Autistic disorder and other pervasive developmental disorders are characterized by symptoms that can include repetitive or compulsive behavior, impaired social interaction, and abnormal language development. These disorders typically manifest in early childhood and result in life-long disability. Serotonin-related brain abnormalities, particularly in autistic disorder, are suggested by reports of low central serotonergic responsivity in this population, altered brain synthesis of serotonin in autistic boys, and more whole-blood serotonin in approximately one-third of affected individuals
(1–
5).
Evidence of serotonergic abnormalities provides a rationale for treatment of autistic disorder or other pervasive developmental disorders with serotonin reuptake inhibitors or selective serotonin reuptake inhibitors (SSRIs). Symptomatic improvement during serotonin reuptake inhibitor or SSRI treatment has been reported in both open-label and controlled studies for most dimensions of clinical impairment associated with autism
(6–
16). One carefully designed treatment study
(7), which evaluated fluvoxamine under double-blind conditions in adults with autistic disorder, found that eight of 15 patients in the fluvoxamine-treated group (in comparison to none of 15 in the placebo group) responded to active drug treatment. Fluvoxamine was superior to placebo in reducing repetitive behaviors, maladaptive behavior, and aggression, as well as in enhancing social relatedness and use of language. Treatment response was not predicted by subjects’ gender, age, severity of autistic behavior, or intellectual functioning.
There has been a substantial increase in the use of SSRIs in pediatric populations, including those with autistic disorder or other pervasive developmental disorders
(17,
18). However, dose-ranging studies of SSRIs and their corresponding pharmacokinetics have not been extensively investigated in this age range. Indeed, current pediatric dosing guidelines for all psychotropic drugs are based on limited blood pharmacokinetics data and are primarily extrapolated from adult dosing studies.
The developing field of magnetic resonance spectroscopy (MRS) allows noninvasive, in vivo brain pharmacokinetic studies of drugs, such as fluvoxamine and fluoxetine, that contain fluorine (
19F) in their native structure
(19).
19F MRS provides an ideal modality for studying brain pharmacokinetics in pediatric patients because it involves no ionizing radiation or special labeling of the compound to be measured. Moreover, it is less motion sensitive than other magnetic resonance imaging techniques, making it clinically feasible for studying individuals who may be unable to remain completely still during examinations.
19F MRS work by our group
(20–
22) has extended the framework of classical pharmacokinetics by applying a multicompartmental model to calculate the steady-state concentration, volume of distribution, and elimination half-life of selected SSRIs in the adult human brain. An emerging
19F MRS technique, magnetization transfer spectroscopy (MTS), has been also used to characterize the interaction between bound and free molecules in vivo as a measure of brain fluoxetine bioavailability in adults
(23). Magnetization transfer effects, generated by the selective preexcitation of the bound fraction of a compound with subsequent transfer of magnetization to the free fraction, cause the partial saturation of the free, or unbound, component. This partial saturation is detected as reduced signal intensity in the MRS pulse sequence. The
19F MTS technique, to our knowledge, has not been used to measure drug bioavailability in children.
The purpose of this study was to use 19F MRS to measure whole-brain SSRI concentrations and demonstrate the feasibility of using 19F MTS to characterize the bound portion of SSRIs prescribed to pediatric patients undergoing treatment for a diagnosis of autistic disorder or other pervasive developmental disorders. Data acquired from this pediatric study group were compared to similarly acquired data from adults prescribed fluvoxamine or fluoxetine for a diagnosis of major depression, obsessive-compulsive disorder (OCD), or panic disorder.
Method
Twenty-one pediatric patients were referred from the Center on Human Development and Disability or the Department of Pediatrics at the University of Washington for participation in the study. Parents or guardians gave informed written consent, and older subjects were given the opportunity to provide informed written assent to participate; both procedures were approved by the institutional review board of the University of Washington. The pediatric group met DSM-IV criteria for autistic disorder or other pervasive developmental disorders through diagnostic evaluation by an experienced, university-based clinician (A.S.U., C.C., or G.D.). All subjects were being treated with either fluvoxamine or fluoxetine and had been taking a consistent daily dose for a minimum of 6 weeks before the study. Exclusion criteria included treatment with other medications, identified genetic conditions in which autistic behavior is symptomatic (e.g., fragile X syndrome), uncontrolled seizures, or contraindications to magnetic resonance scanning, such as metal implants or claustrophobia. Parents or guardians were given the option of administering oral diphenhydramine, 25–50 mg, for light sedation 1–2 hours before the study if their child had previously demonstrated a sedative response to this medication.
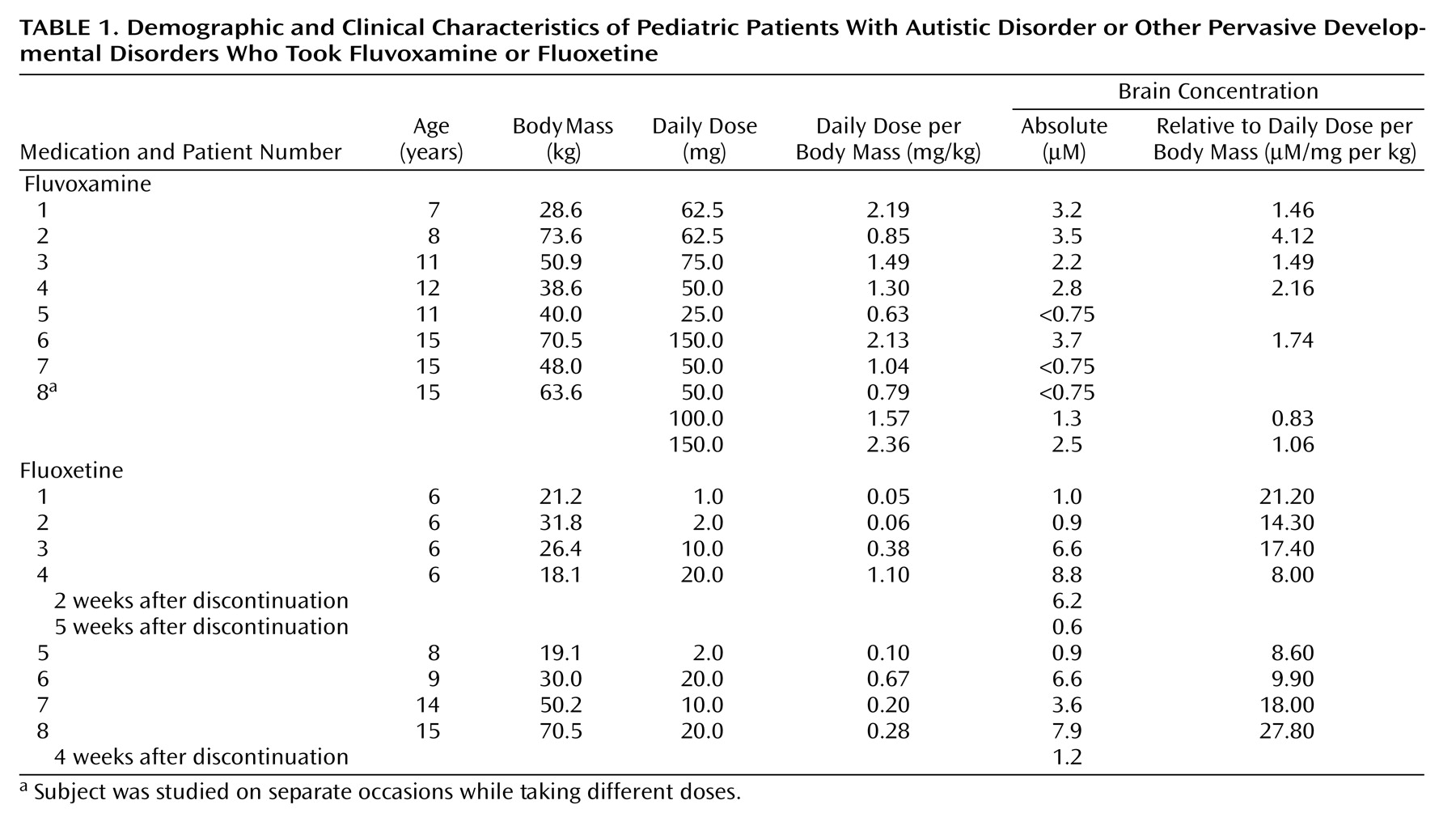
Of the 21 pediatric subjects, 16 successfully completed the brain drug concentration measurement protocol, while five of the subjects were not cooperative with the study. Eight of the subjects (mean age=11.8 years, SD=3.2, range=7–15) were taking fluvoxamine at a mean dose of 77.5 mg/day (SD=42.8, range=25–150) at a dose-per-body-mass ratio of 1.43 mg/kg (SD=0.62). One subject was studied on three separate occasions while taking progressively higher doses of fluvoxamine (50 mg/day, 100 mg/day, and 150 mg/day, each at a consistent dose for more than 1 month); each measurement was treated as an independent data point. Eight of the subjects (mean age=8.8 years, SD=3.7, range=6–15) were taking fluoxetine at a mean dose of 10.63 mg/day (SD=8.5, range=1–20), at a dose-per-body-mass ratio of 0.36 mg/kg (SD=0.36). Two subjects who were studied while taking fluoxetine were then restudied after cessation of drug therapy.
With use of identical
19F MRS methods, brain concentrations from outpatients under treatment at the University of Washington Center for Anxiety and Depression for a DSM-IV diagnosis of panic disorder, major depression, or OCD were determined for 13 adults (mean age=44.8 years, SD=17.5, range=18–66) who were taking fluvoxamine at a mean stable dose of 231 mg/day (SD=69, range=100–300) for an average treatment duration of 7.5 months (SD=4.2, range=2–24) and 15 adults (mean age=51.9 years, SD=11.3, range=24–62) who were taking fluoxetine at a mean stable dose of 24.0 mg/day (SD=7.4, range=20–40) for an average treatment duration of 11.3 months (SD=6.9, range=3–24). Steady-state brain fluvoxamine and fluoxetine concentrations for these adults have, in part, been previously published
(20–
23). Data from one adult subject previously identified as having a metabolic abnormality on the basis of consistent abnormally high (∼fivefold) brain levels of fluvoxamine
(20) were not included in these analyses.
Determination of whole-brain drug concentrations were performed by using previously published methods
(20,
21). In brief, all data were acquired by using a GE Signa 1.5-T magnetic resonance scanner (General Electric Medical Systems, Milwaukee) with version 5.8 software and equipped with a broad-band power amplifier (General Electric Medical Systems, Milwaukee) and a fluorine quadrature birdcage head coil built in our laboratory
(23). A T
1-weighted proton image was acquired with the fluorine coil to ensure proper positioning of the subject’s head in the magnet. Whole-brain shimming of the magnetic field, to ensure consistent magnetic field homogeneity, was performed through the proton channel, since the fluorine signal is too weak to allow adequate shimming. The
19F MRS pulse sequence was set at 90°, with a pulse width of 500 μsec, a phase cycling of 2, and TR of 1 second to obtain a fully relaxed spectrum measurement with about 1,000 averages. Power for the 90° pulse was determined by using a sodium fluoride phantom and a resistive device designed to load the coil similarly to a human head. Drug concentration was determined relative to the phantom. Total scanning time was generally less than 40 minutes. Signal quantification was performed by using a VARPRO-based time-domain fitting program (MRUI, Delft, the Netherlands). Aged-adjusted literature values for brain volume were used as a correction factor for calculating brain concentrations, since it was not feasible to measure individual brain volumes because of technical limitations using the fluorine coil to acquire high-resolution proton images
(24,
25).
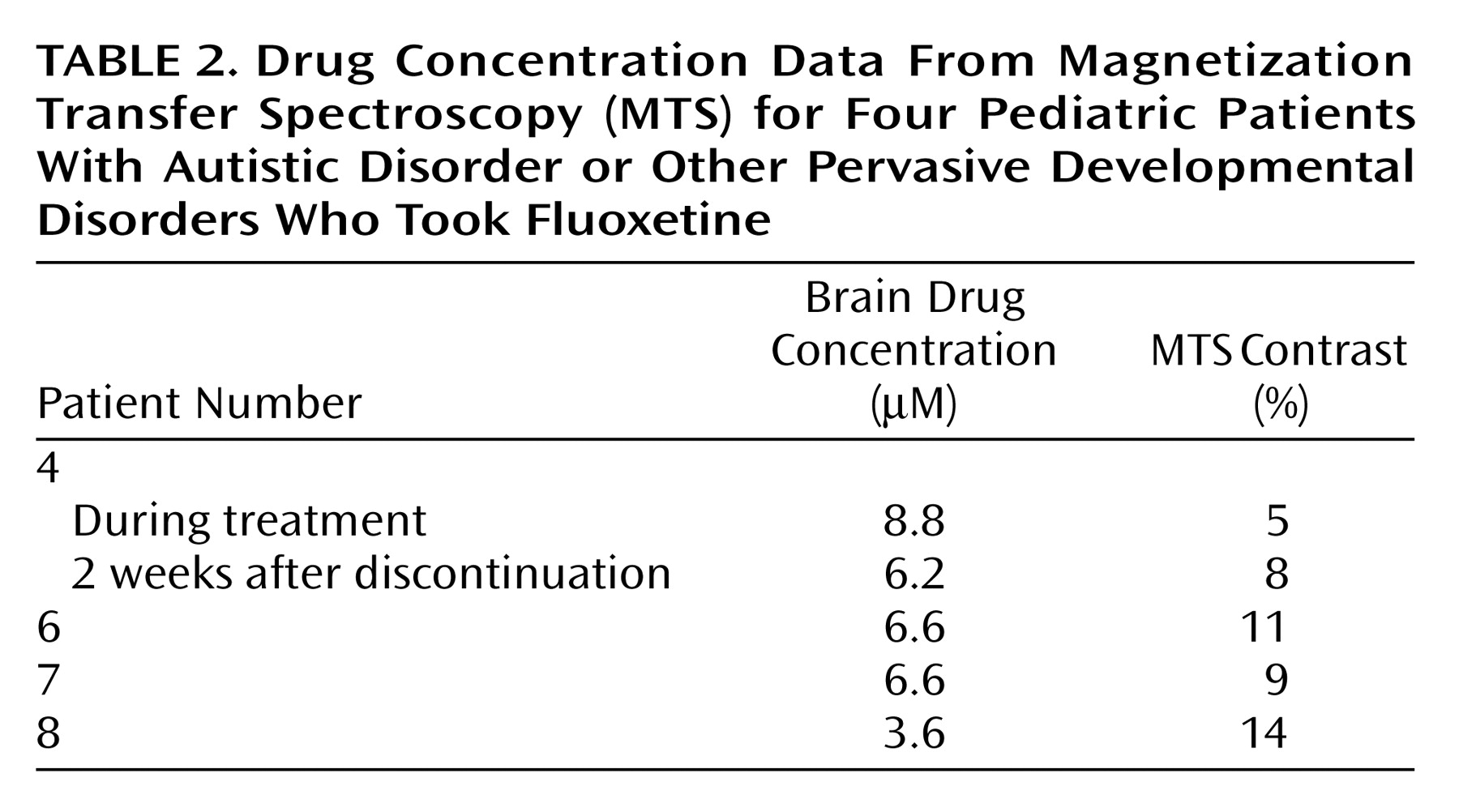
MTS was performed to characterize brain drug binding for a subgroup of four pediatric subjects taking fluoxetine. Data are included for one subject studied in the steady state and 2 weeks after drug discontinuation. The MTS methods were identical to those used in a previously published study of adults
(23). In brief, the MTS pulse sequence consisted of a 90° hard pulse of 500-μsec duration and 0.5-second TR, with and without a train of three saturation pulses centered at –3000 Hz and 0.2 msec before the 90° pulse. The magnitude of MTS contrast was calculated as a signal depression with the preparation pulses.
Statistical handling of the data employed independent t tests to compare pediatric and adult data for each medication separately; assumptions of equal variance were met. Additional analyses were performed by using Pearson’s r to evaluate the relationship between age and brain measurements for each medication across the age range studied. All data are presented as means and standard deviations.
Results
A whole-brain fluorine signal could be quantified for all subjects, with the exception of three pediatric patients who were taking fluvoxamine at doses between 25 and 50 mg/day and who had
19F MRS signals below the level of quantifiable detection (<0.75 μM). Individual pediatric subjects’ brain concentrations of fluoxetine and fluvoxamine, also calculated in relationship to dose per body mass (mg/kg), are shown in
Table 1.
The dose-per-mass relationship for the fluvoxamine prescriptions was lower in the pediatric group than in the adult group (mean=1.43 mg/kg, SD=0.62, versus mean=2.94 mg/kg, SD=0.78) (t=5.02, df=21, p=0.001). Consistent with this, the average brain fluvoxamine concentration in the pediatric subjects was lower than that observed for adults (mean=2.74 μM, SD=0.83, versus mean=7.22 μM, SD=2.66) (t=4.29, df=18, p=0.001). When brain concentration was corrected for the effects of dose per mass, the ratio of the concentration per dose per mass in the pediatric group was not significantly different from that for the adults (mean=1.91 μM/mg per kg, SD=1.03, versus mean=2.56 μM/mg per kg, SD=0.71) (t=1.68, df=18, p=0.11).
The dose-per-mass relationship between the pediatric and adult subjects did not differ significantly for fluoxetine prescriptions (mean=0.36 mg/kg, SD=0.36, versus mean=0.31 mg/kg, SD=0.14) (t=0.40, df=21, p=0.69). Correspondingly, the average brain concentration of fluoxetine was similar between the two age groups (mean=4.54 μM, SD=3.33, versus mean=5.04 μM, SD=2.54) (t=0.41, df=21, p=0.69). When corrected for dose per mass, the brain ratios for concentration per dose per mass remained similar between the groups (mean=15.66 μM/mg per kg, SD=6.89, versus mean=17.49 μM/mg per kg, SD=9.56) (t=0.48, df=21, p=0.64).
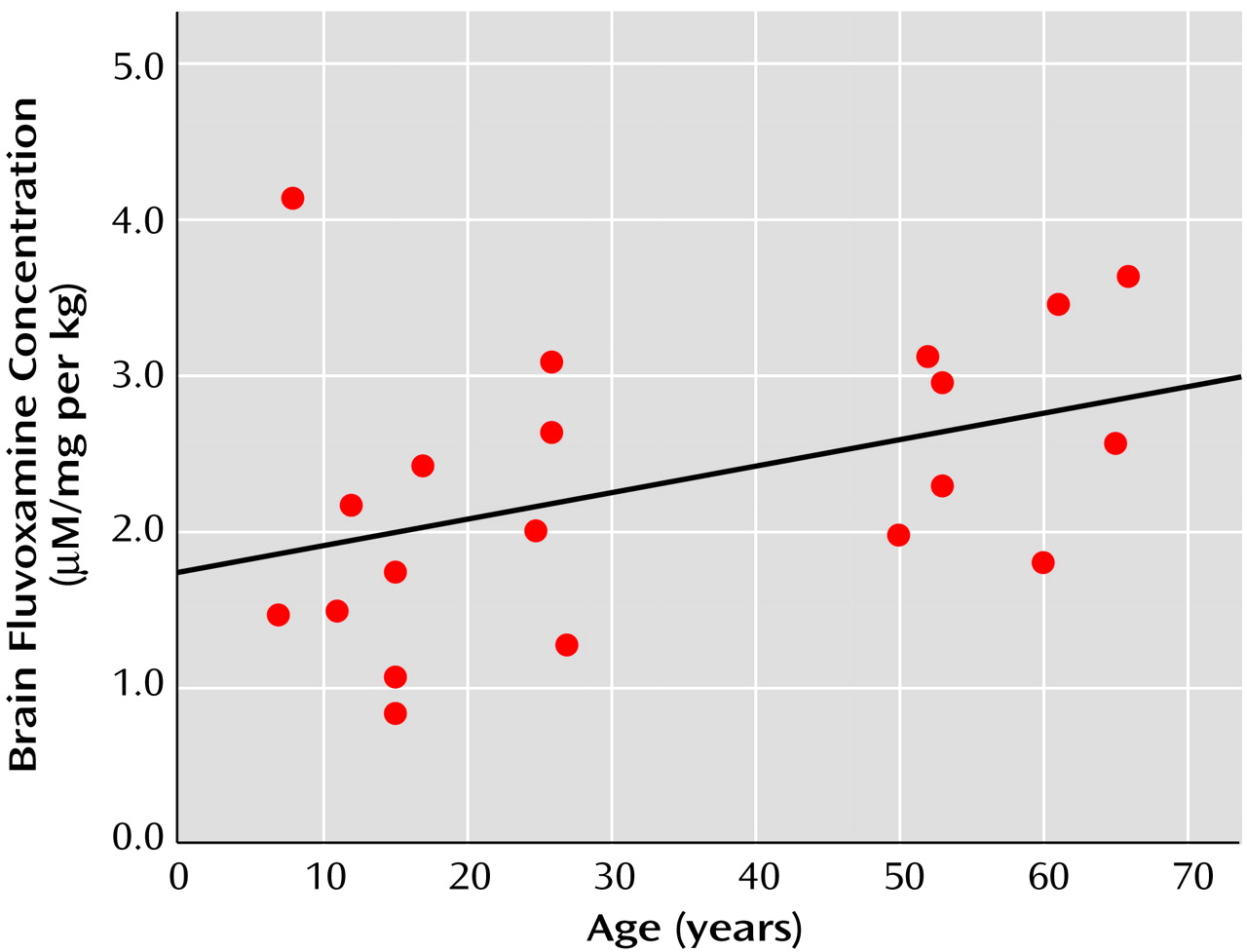
A significant correlation between brain concentration and dose of medication was found both for fluvoxamine (r=0.71, df=20, p=0.001) and fluoxetine (r=0.46, df=23, p=0.03). For fluvoxamine, a positive correlation observed between age and brain concentration (r=0.52, df=20, p=0.02) no longer reached significance when brain fluvoxamine concentration was adjusted for the effects of dose per mass (r=0.41, df=20, p=0.08). For the combined pediatric and adult groups, this relationship between brain fluvoxamine concentration per dose per mass and age is shown in
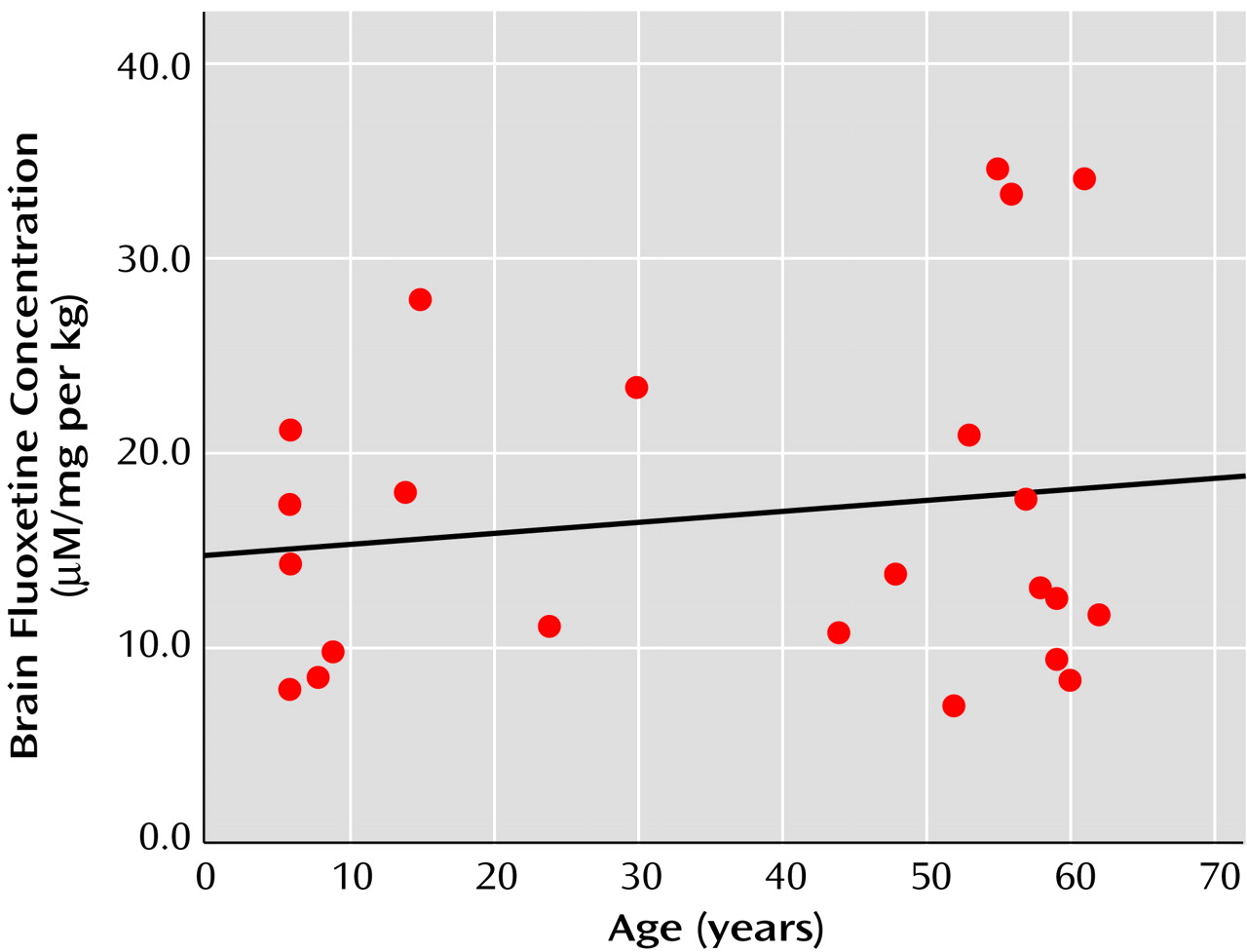
Figure 1. No relationship was found between age and brain fluoxetine concentration (r=0.12, df=23, p=0.55), nor was there a relationship between age and the ratio for brain concentration per dose per mass (r=0.15, df=23, p=0.50), as shown in
Figure 2.
Fluoxetine data were acquired after discontinuation of the medication by two pediatric subjects. Brain fluoxetine concentration had fallen by 93% at 5 weeks after discontinuation of medication for one subject (subject 4), and there was an 85% decrease in brain fluoxetine concentration for the second subject (subject 8) 4 weeks after discontinuation of the medication.
19F MTS demonstrated a quantifiable contrast for the fluoxetine signal (mean=9.4%, SD=3.4%, range=5%–14%) (
Table 2). There was a significant negative correlation observed between brain fluoxetine concentration and the magnitude of MTS contrast (r=–0.92, df=5, p<0.03). In a comparison of MTS contrast for fluoxetine between data from the pediatric age group and the previously determined adult values (mean=12.5%, SD=5.0%) (23), no significant difference was observed (t=1.2, df=9, p=0.27).
Discussion
To our knowledge, this is the first study to systematically evaluate the effects of age on brain steady-state concentrations of SSRIs in humans. Our findings, from a group of pediatric patients with a diagnosis of autistic disorder or other pervasive developmental disorders, suggest that pediatric brain levels of fluvoxamine and fluoxetine are not significantly different from typical adult levels when corrected for the effects of dose per mass. Overall, in a direct comparison of the two age groups, and in a combination of the age groups for evaluation across an extended age range, from childhood to later adulthood, no clear relationship was observed between age and brain SSRI concentrations after adjustment for the effects of dose per body mass. These findings suggest that therapeutic dosing of fluvoxamine and fluoxetine can be extrapolated in relationship to dose per body mass from adult treatment practices and can provide an acceptable treatment approach for the medication management of this pediatric population.
There was found a significant effect of age on brain concentration for fluvoxamine, but not fluoxetine; lower brain levels of fluvoxamine were observed in the pediatric patients. However, differences between age groups in brain fluvoxamine concentration on a dose-per-body mass basis were not significant, although a weak, nonsignificant correlation with age remained. It is likely that any age relationship to brain concentration of fluvoxamine primarily reflects the lower dose-per-mass equivalent prescribed for the pediatric patients than for adults, a difference not observed for fluoxetine. Age-related differences in dosing patterns between the two drugs probably reflect their respective side effect profiles in pediatric populations.
The elimination half-life of fluoxetine and its metabolites from the adult brain has been estimated, based on limited observations, to be approximately 16 days or longer
(26,
27). The rate of brain clearance of fluoxetine and its metabolites that was observed for the two pediatric subjects in this study suggests that the brain elimination half-life of fluoxetine is not prolonged in this age group. Our preliminary findings of a similar brain elimination time course for pediatric subjects as has been observed for adults after fluoxetine discontinuation are consistent with the similar brain concentrations achieved between age groups on a dose-per-body mass basis. However, from the small number of subjects studied, we cannot exclude the possibility that the kinetics of drug uptake or clearance in the brain may vary as a function of age until additional investigations are carried out to systematically address these issues.
Age-related effects on the plasma pharmacokinetics of antidepressants, which typically are metabolized more rapidly by children and more slowly by older adults, have provided a clinical rationale for upward dose adjustments for children that may sometimes far exceed adult daily doses prescribed on a dose-per-mass basis
(28). Hepatic clearance of medications is more efficient in children because of the disproportionate size of their livers relative to body size. However, fluvoxamine and fluoxetine are preferentially distributed from plasma into brain tissue because of their high lipophilicity, which results in approximately 20-fold higher brain concentrations that would substantially lessen the impact of hepatic clearance on steady-state brain levels
(22). The substantial concentration gradient between brain and plasma may also account for observations that combined plasma fluoxetine and norfluoxetine concentrations, when adjusted for dose-per-mass relationships, are similar between pediatric and adult populations (unpublished data from Eli Lilly and Company).
Prior work making use of MTS in conjunction with
19F MRS quantification of fluoxetine in the adult brain
(23) has demonstrated the use of this technique to better understand brain bioavailability of psychotropic drugs. These preliminary
19F MTS findings in pediatric patients are consistent with work in adults
(23) that found an inverse relationship between brain drug concentration and MTS contrast. This inverse relationship implies saturable binding of fluoxetine in both pediatric and adult brains; the bioavailability of the drug is proportional to brain concentration.
Several potential limitations of the study design may have influenced our results. For example, the time to reach brain steady state in pediatric patients has not been established for either fluvoxamine or fluoxetine; inclusion criteria for minimum duration of treatment were extrapolated from the available adult literature. While the time course for brain steady state of fluvoxamine has been determined to be less than 30 days in adults
(20), a substantially longer duration of treatment may be required to achieve brain steady state for fluoxetine
(29) and longer than the minimum criteria of 6 weeks at consistent medication doses that was met by two of the children studied. Furthermore, this study investigated brain concentrations of fluvoxamine and fluoxetine only in pediatric patients diagnosed as having autistic disorder or other pervasive developmental disorders. As there is evidence to suggest both brain structural and brain chemical abnormalities in younger-aged children with these disorders
(30,
31; unpublished data of Dager et al.), it is uncertain how generalizable our findings are to other pediatric populations undergoing treatment with SSRIs. An additional consideration is that individual brain volume measurements were unavailable for the subjects in this study. If the pediatric group had greater brain volumes, reported to be approximately 10% larger among a group of 3–4-year-old children with autistic disorder or other pervasive developmental disorders
(31), then we would have overestimated brain drug concentrations by using typical age-adjusted values for brain volumes. However, this is an unsettled issue, since recent findings suggest that early brain enlargement may occur from accelerated brain growth among very young children with autism, which plateaus at about 5 years of age
(30) and before the minimum age of entry into this study.
Findings from this study may help to provide a brain pharmacokinetic basis for current dosing strategies in prescribing SSRIs to pediatric patients. We found that children and adolescents with autistic disorder or other pervasive developmental disorders attained similar brain levels of fluvoxamine and fluoxetine on a dose-per-body mass basis, as did adults treated for anxiety disorders or major depression. Thus, a reasonable target dose range for prescribing fluvoxamine and fluoxetine in pediatric populations may be determined by scaling adult doses in relationship to body mass.