It has been hypothesized that information processing mechanisms are impaired in schizophrenia patients. One of the mechanisms of information processing is the ability to filter or “gate” information. Gating is critical for the brain’s capacity to maintain integrity of thought and behavior
(1,
2). Prepulse inhibition of the human startle reflex offers an operational measure of sensorimotor gating
(1–
5). In brief, prepulse inhibition occurs when a weak prestimulus presented 30 to 500 msec before a startling stimulus results in a decrease in the startle reflex. Prepulse inhibition is regulated by forebrain cortico-striato-pallido-thalamic-mediated plasticity (e.g., gating) of the startle response. Furthermore, prepulse inhibition is useful in schizophrenia research because a variety of pharmacological, neuroanatomic, and psychopathologic factors can modify the gating of the startle response
(1–
5).
Braff and colleagues
(3) first reported that schizophrenia patients have prepulse inhibition deficits. Since then, numerous studies have replicated their finding
(6–
14). Additionally, Cadenhead and colleagues have demonstrated prepulse inhibition impairments among schizotypal patients
(15), most of whom were unmedicated, and first-degree relatives of schizophrenia patients
(16). A diminution of prepulse inhibition also occurs in animals administered dopamine agonists, such as amphetamine and apomorphine, and in animals in which the cortico-striato-pallido-thalamic circuitry has been experimentally altered
(17). Furthermore, these pharmacologically induced prepulse inhibition deficits are reversed by antipsychotic medications
(18). Collectively, these studies have been the basis for the suggestion that prepulse inhibition is useful in investigating antipsychotic efficacy for the treatment of schizophrenia
(8,
18).
There is growing evidence that gating deficits are related to cognitive fragmentation, which may manifest in the form of thought disorder. Perry and Braff
(9) demonstrated that schizophrenia patients with the lowest levels of prepulse inhibition exhibited the poorest performance on thought disturbance indices. In a follow-up experiment, Perry and colleagues
(4) demonstrated that the correlation between prepulse inhibition deficits and thought disorder is extremely robust when the patient is assessed for thought disorder during the assessment of prepulse inhibition.
Recently, it has been reported that prepulse inhibition deficits may be responsive to and at least partially reversed at short prepulse inhibition intervals by treatment with atypical antipsychotics
(8,
12,
22). It has also been shown that schizophrenia patients treated with typical antipsychotics had prepulse inhibition levels that did not significantly differ from those of healthy comparison subjects. Similarly, Kumari and colleagues
(22) found that early onset of illness was associated with prepulse inhibition deficits, which did not improve with atypical antipsychotic treatment as it did in those patients with later onset. They suggested that “age of illness onset may be a moderating variable in the disruption of prepulse inhibition” (p. 612). Collectively, these studies support a hypothesis that prepulse inhibition deficits are trait-related in schizophrenia patients but also vary (as do symptoms such as thought disorder) within and between schizophrenia patients.
These preliminary findings support the position that antipsychotic medication mediates prepulse inhibition. However, several of these studies compared stable patients with low psychosis rating scores to more acutely ill patients. Thus, it remains unclear whether these prepulse inhibition findings are due to the direct pharmacological impact of antipsychotics or to antipsychotic-related symptom reduction.
In order to begin to compare the contribution of symptom level versus medication status to prepulse inhibition and habituation, we assessed two groups of schizophrenia patients as well as a group of healthy comparison subjects. Both schizophrenia patient groups had been admitted to an inpatient psychiatric facility because of acute psychosis. One group had been unmedicated at the time of admission and remained unmedicated during prepulse inhibition testing; the second patient group had been receiving antipsychotic medication at the time of admission and the prepulse inhibition testing. On the basis of previous findings
(8,
12,
14,
22), we predicted that both groups of schizophrenia patients would have lower levels of prepulse inhibition and startle habituation than would the healthy comparison subjects. We also hypothesized that there would be no prepulse inhibition or startle habituation differences between the acutely psychotic schizophrenia patients who were medicated and those who were unmedicated at the time of admission, since it seems likely that the cortico-striato-pallido-thalamic circuitry is dysregulated, thus resulting in both prepulse inhibition deficits and increased psychotic symptoms. Finally, we explored whether an association between age at onset of illness and prepulse inhibition would be present among the acutely psychotic patients with early-onset schizophrenia.
Results
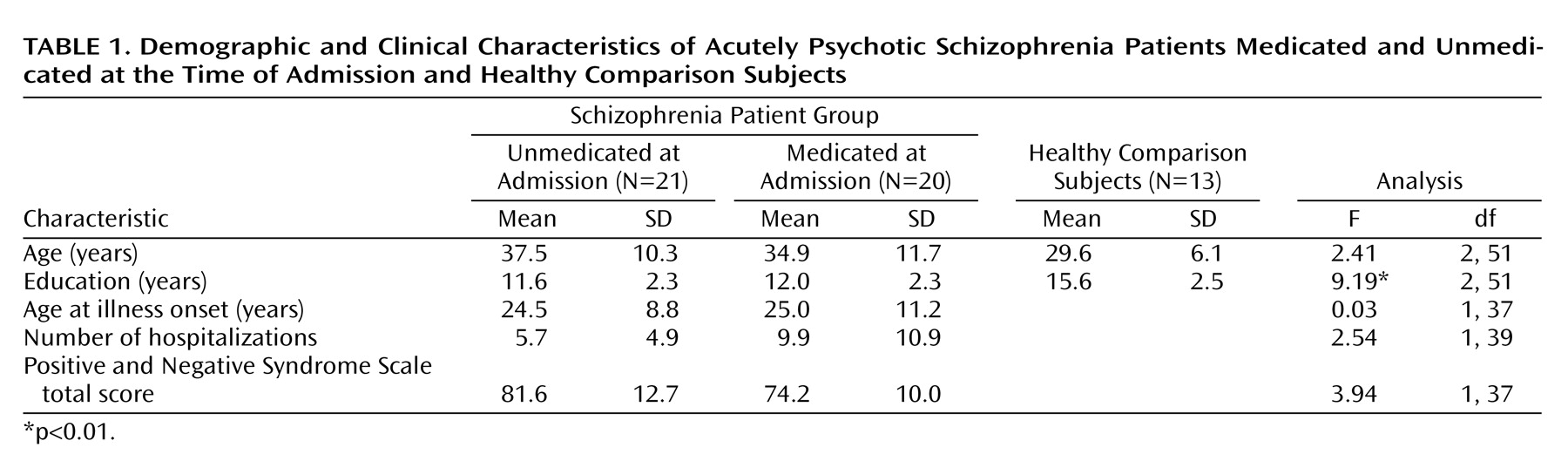
The healthy comparison subjects had significantly more years of education than did the schizophrenia patients (
Table 1) but were not significantly different in age nor were there differences in gender distribution among the groups. There were no statistically significant differences between the two schizophrenia patient groups for age, years of education, age at onset of illness, or number of hospitalizations. The patients who were unmedicated at the time of admission had higher Positive and Negative Syndrome Scale scores than those who had been medicated, a difference that approached statistical significance (F=3.94, df=1, 37, p=0.06) (
Table 1).
Responsivity and Startle Magnitude
There were no healthy comparison subjects excluded because of startle nonresponsivity. One schizophrenia patient from the unmedicated at admission group was excluded because of a lack of measurable startle response, whereas seven patients from the medicated group were startle nonresponders. A comparison of the number of schizophrenia patients excluded from the final analysis because of startle nonresponsivity revealed that significantly more of the medicated subjects than unmedicated subjects were excluded (p<0.05, Fisher’s exact test).
Startle response magnitude to the pulse-alone conditions did not significantly differ among the three groups (F=1.91, df=2, 51, p=0.16).
Prepulse Inhibition
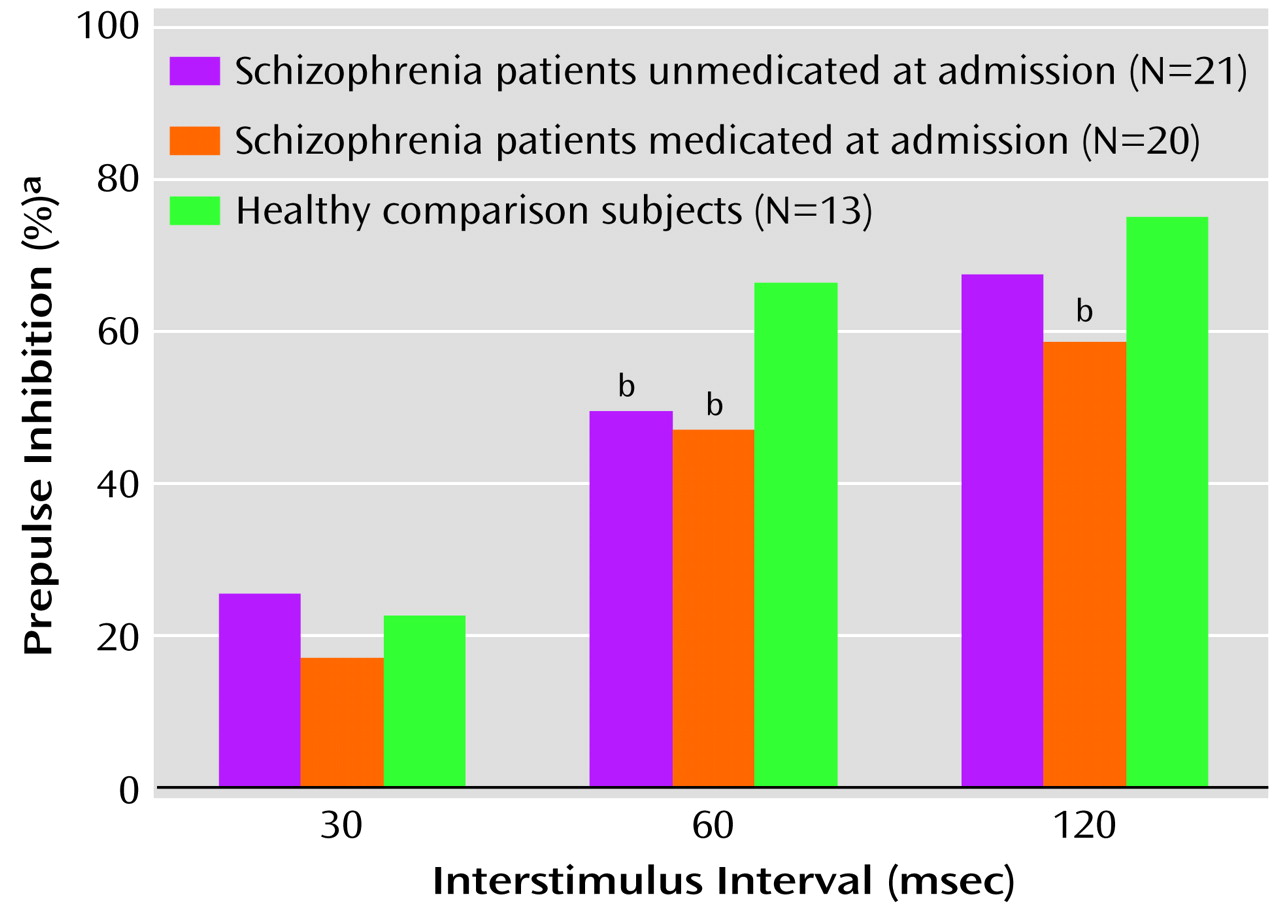
Examining normality and homogeneity of variance revealed a highly skewed (range=–1 to –3) distribution of scores as well as heterogeneous variance among the groups. Therefore, the Kruskal-Wallis test was used to analyze the data. There was no difference in rankings for the 30-msec prepulse condition (χ2=0.98, df=2, p=0.62) among the three groups. There was a significant difference in rankings between the healthy comparison subjects and the schizophrenia patients in the 60-msec prepulse condition (χ2=7.02, df=2, p<0.05) and the 120-msec prepulse condition (χ2=9.89, df=2, p<0.05). Follow-up comparisons revealed that patients unmedicated at admission significantly differed from the healthy comparison subjects in the 60-msec condition (Mann-Whitney U=60, p<0.01), and the difference in the 120-msec condition approached significance (Mann-Whitney U=83.5, p=0.06). Patients medicated at admission significantly differed from the healthy comparison subjects in the 60-msec condition (Mann-Whitney U=78, p<0.05) and the 120-msec condition (Mann-Whitney U=43, p<0.01).
To compare the two schizophrenia patient groups across the three different prepulse inhibition conditions, Mann-Whitney U tests were again used and revealed no significant differences (30-msec condition: U=174, p=0.35; 60-msec condition: U=195, p=0.70; 120-msec condition: U=160, p=0.19). The median prepulse inhibition values for the three groups are illustrated in
Figure 1. For the patients medicated at the time of admission, percent prepulse inhibition for each of the interstimulus interval conditions was independently examined by medication type; there were no significant differences noted between subjects receiving typical antipsychotics versus risperidone or olanzapine.
Spearman rank-order correlation analyses revealed no significant associations between any of the prepulse inhibition intervals and any of the Positive and Negative Syndrome Scale scores. When the analyses were rerun for the two patient groups independently, there were still no significant correlations between prepulse inhibition and the Positive and Negative Syndrome Scale scores.
Subjects were divided according to age at onset into two groups independent of medication status. Age at onset information was unavailable for two of the 41 patients. The early-onset group (onset of illness at age 20 or younger, selected according to the procedure used by Kumari et al.
[22]) consisted of 16 patients; the later-onset group (onset of illness at age 21 or older) consisted of 23 patients. There was no statistical difference between the schizophrenia patient groups on prepulse inhibition across the three interstimulus intervals. There was a statistically significant difference between the early-onset patients and healthy comparison subjects for the 60-msec condition (U=58, p<0.05) and the 120-msec condition (U=53, p<0.05). The later-onset patients also significantly differed from the healthy comparison subjects in both the 60-msec condition (U=77, p<0.05) and the 120-msec condition (U=72.5, p<0.05). Finally, there were no statistically significant correlations between prepulse inhibition and age at onset of illness when all schizophrenia patients were grouped together. When the groups were divided into early and later onset, however, there was a significant positive correlation between age at onset and prepulse inhibition at the 30-msec condition for the later-onset patients only (r
s=0.53, N=23, p<0.01).
Startle Habituation
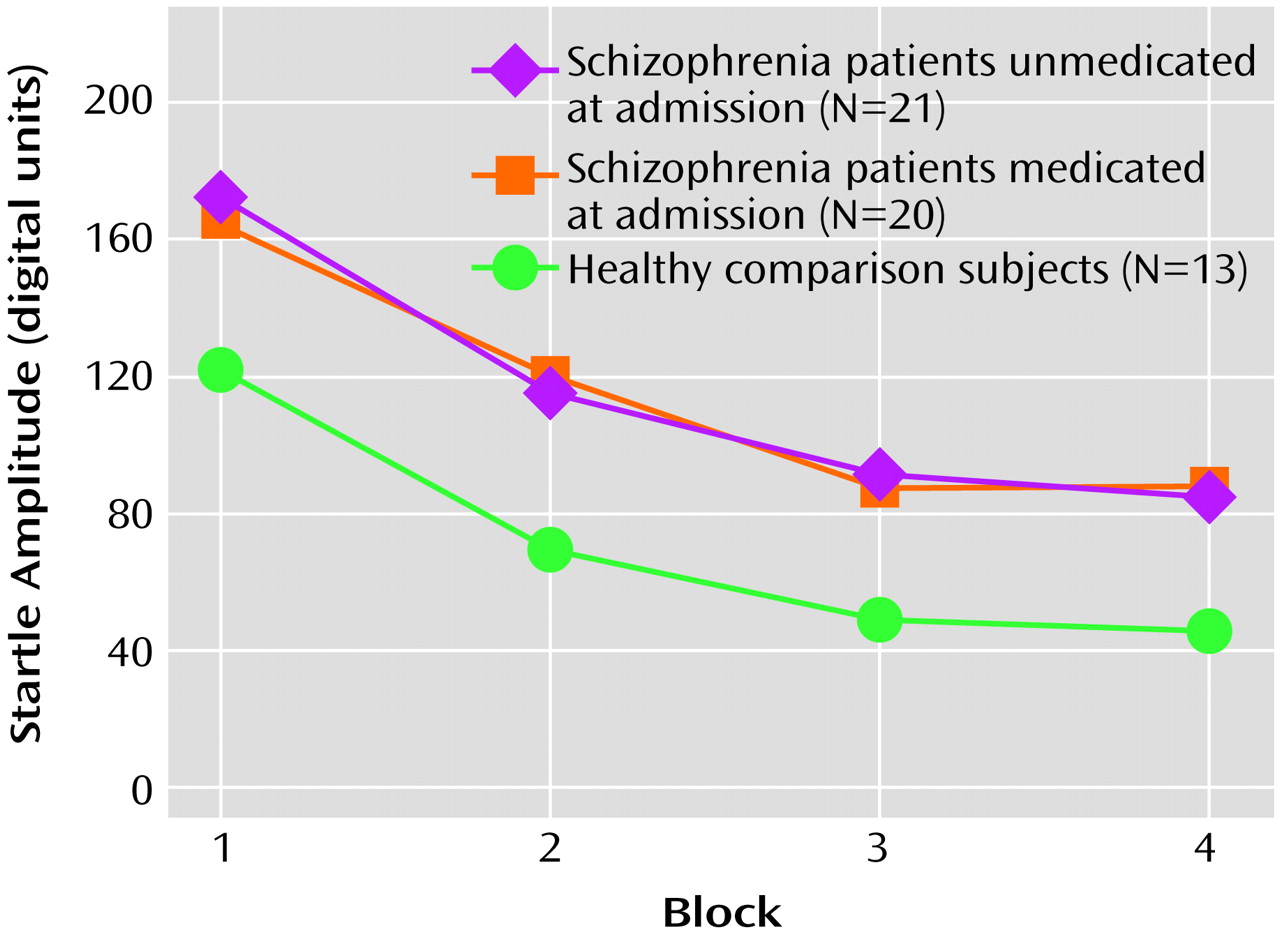
Analysis of the startle habituation data revealed that there was an overall significant main effect for block (F=39.98, df=3, 49, p<0.001), reflecting habituation within all three groups (
Figure 2). There was no main group effect nor an overall block-by-group interaction (F=0.82, df=6, 100, p=0.56). The percent habituation from block 1 to block 4 was 50.5% for the patients unmedicated at admission, 50.7% for the medicated patients, and 63.6% for the healthy comparison subjects. Planned multiple comparisons revealed that the healthy subjects had greater habituation relative to the patients unmedicated at admission (t=1.81, df=32, p=0.07) and the medicated patients (t=1.90, df=31, p=0.07); these differences approached statistical significance. Within the schizophrenia patient group, there were no significant differences between those who were unmedicated at admission and those who had been receiving medication (t=0.03, df=39, p=0.98).
Discussion
In the present study, three groups were examined on various startle measures. The results reveal that there were no differences between the three groups in startle magnitude. Significant differences, however, were detected between schizophrenia patients and healthy comparison subjects in two of the three prepulse inhibition conditions (interstimulus intervals of 60 and 120 msec). This finding replicates previous findings that have demonstrated deficits in prepulse inhibition among schizophrenia patients versus healthy comparison subjects
(3,
6,
7,
10,
11,
13,
14). Within this group of acutely psychotic schizophrenia patients, there were no statistically significant differences noted between those who were unmedicated at the time of admission and those who had been receiving medication on measures of startle reactivity, prepulse inhibition, or startle habituation. Given that the study group sizes were small, however, these findings should be considered preliminary and requiring of replication in a larger sample.
In contrast to the present findings, Weike and colleagues
(12) reported greater prepulse inhibition deficits among unmedicated schizophrenia patients versus medicated patients. In that study, the unmedicated group consisted of patients who were tested immediately following hospital admission and before antipsychotic treatment was started (similar to the present design); additionally, five of these patients were having their first psychotic episode and had never been medicated before. They contrasted this unmedicated patient group with 20 patients who had been stabilized after 11 weeks of neuroleptic treatment (11 had received typical antipsychotic medications, and nine had been given atypical antipsychotics). The unmedicated group exhibited significantly more positive symptoms than did the medicated group. This difference in symptom status may have contributed to the prepulse inhibition differences. Furthermore, they found that lower levels of prepulse inhibition were correlated with higher levels of positive symptoms, consistent with what we have previously reported
(4,
7,
9). Those authors, however, concluded that impaired prepulse inhibition is not stable and may be improved by neuroleptic treatment.
Kumari et al.
(8) tested two groups of stable schizophrenia patients that had similar Positive and Negative Syndrome Scale scores. In their cross-sectional study, these authors found that 11 patients treated with clozapine had higher levels of prepulse inhibition than did nine patients treated with other typical antipsychotic medications. These differences were significant at the briefer interstimulus intervals only (30 and 60 msec). They also found that the levels of prepulse inhibition of the clozapine-treated patients were similar to those of healthy comparison subjects. Kumari and colleagues concluded that normal “attentional and information processing functions may be attributable to the cognitive enhancing characteristics of atypical antipsychotics” (p. 1049). In the present study, when we examined prepulse inhibition by medication type (atypical versus typical antipsychotic), we did not find a significant difference in prepulse inhibition levels. Clearly future studies with larger sample sizes are needed to understand the impact of antipsychotic medications on prepulse inhibition.
One possibly important difference in this study compared with the work of Weike et al.
(12) and Kumari and colleagues
(8) is that our patients had symptom rating scores that were approximately twice as high as those reported in these other two studies. Similar to our findings, Parwani and colleagues
(14) reported prepulse inhibition deficits in schizophrenia patients versus healthy comparison subjects. The patients in the Parwani et al. study consisted of inpatients and outpatients who had high symptom rating scores, similar to the acutely psychotic subjects in our study. They concluded that prepulse inhibition deficits reflect an enduring abnormality in schizophrenia that is unrelated to clinical state.
The hypothesis of a relationship between age at onset of illness and prepulse inhibition
(22) was not supported. In the present study, statistically significant correlations between prepulse inhibition and age at onset of illness were found only in one condition (the 30-msec interstimulus interval) for patients with later-onset schizophrenia. There was no significant correlation when the patients were combined into a single group. Furthermore, unlike the findings of Kumari et al.
(22), we did find that both the early- and late-onset groups demonstrated significant differences in prepulse inhibition when compared with healthy subjects.
There are several limitations and caveats in the present study that should be highlighted. First, all but three of the unmedicated patients had been previously treated with antipsychotic medication; therefore, there was substantial variation regarding the last time that the subject was exposed to medication. Second, it is well-known that although D
2 antagonism can be achieved with a single administration of an antipsychotic, the full clinical effects (including cognitive stabilization) often take weeks
(29). Therefore, dividing subjects on the basis of short-term medication status may not fully address the role of antipsychotic medication upon the neural substrate of prepulse inhibition. Last, and most important, although patients in our medicated group were being treated with antipsychotic medication and reportedly had been taking medication immediately before admission, we did not use serum antipsychotic levels to verify their status, since this strategy is fraught with difficulties in a noncontrolled setting. Therefore, we have no systematic way of measuring their medication compliance before hospitalization, and thus we may have some patients who overreported their compliance. We did, however, confirm medication compliance in 80% of the patients through direct contact with caretakers and significant others. Future studies will need to consider prior exposure as well as current medication status when interpreting prepulse inhibition results. Still, the most parsimonious explanation of our preliminary results is that prepulse inhibition deficits are less dependent upon medication status during severe psychotic states. This is consistent with our previous finding and resulting “model” that prepulse inhibition was highly associated with thought disturbance levels independent of medication
(4,
7,
9). Thus, it appears that both the neural circuitry underlying prepulse inhibition and psychosis may be dysregulated during acute psychotic states when the patient is in the early phases of (or requires increases in) medication treatment.
There was no overall significant finding in habituation for our three groups. Startle amplitude, however, decreased 64% in the healthy comparison subjects over the entire session, which was greater than that of the 51% for the schizophrenia patients. This difference, although not reaching statistical significance, is in the predicted direction, consistent with previous findings. Furthermore, the percentage of habituation reported for both groups of patients in the present study is similar to that recently reported by Parwani et al.
(14) and is consistent with Geyer and Braff’s report of habituation deficits in schizophrenia patients treated with typical antipsychotics
(19).
An additional finding was the higher number of subjects that were excluded for being “startle nonresponsive” in the medicated versus unmedicated group. In medicated schizophrenia patient groups, startle nonresponsivity often approaches 25%
(6,
7). Our findings suggest that exclusion may be related to the antipsychotic medication decreasing or blunting startle reactivity. Clearly, future studies need to assess patients longitudinally at baseline and later when they are no longer in the midst of their acute psychosis in order to better understand the role of antipsychotic medication on sensorimotor gating.
In summary, the results of this study add to the literature on information processing and suggest that prepulse inhibition and habituation in schizophrenia patients are not solely influenced by antipsychotic medications when patients are acutely psychotic. In fact, it appears that during acute states, the underlying neural substrate dysfunction that is linked to prepulse inhibition and habituation deficits as well as psychosis is not fully reversed over the course of several days of medication treatment. This observation is consistent with both clinical lore and neurobiological research regarding the attenuation of acute psychotic states in schizophrenia patients. The present findings underscore the need to assess patients longitudinally, when they are acutely symptomatic and not receiving medications and again when they are in relative remission and receiving medication treatment.