The human 5-HT
2A receptor gene has been regarded as a candidate gene for susceptibility to schizophrenia
(1), although there are inconsistent findings
(2,
3). Studies regarding associations between this gene’s polymorphisms and response to clozapine (the prototype of atypical antipsychotics) also yielded conflicting results
(3–
5). Risperidone is a newer atypical agent with efficacy for both positive and negative symptoms of schizophrenia. Its affinity in vitro is 20 times higher for 5-HT
2A receptors than for D
2 receptors; in vivo it occupies 5-HT
2A receptors at a rate 10 times lower than it does D
2 receptors
(6) Clozapine differs from risperidone in its relatively weak 5-HT
2A antagonism, its very weak D
2 antagonism, and its rather high affinities for several other receptors
(6). A balance of full 5-HT
2A receptor occupancy and partial dopamine D
2 receptor occupancy probably underlies the beneficial therapeutic effect of risperidone, especially on negative symptoms
(6). However, the contribution of 5-HT
2 activity to risperidone efficacy is still debatable
(7). The present study investigates the influence of the 102-T/C polymorphism in the 5-HT
2A receptor gene on risperidone’s effectiveness.
Method
All newly hospitalized Han Chinese patients with acutely exacerbated schizophrenia entered this study if they satisfied inclusion criteria described elsewhere
(8). After a complete description of the study to the subjects, written informed consent was obtained.
The subjects initially received a placebo for 1–7 days. Administration of risperidone was then titrated to the target dose of 6 mg/day (or lower, in the case of intolerance) within 1 week. From day 8 to day 42 of risperidone treatment, the dose remained the same as that used on day 7 or could be reduced on day 14 or day 28 to curtail side effects. This dosing strategy was based on our recent work
(8). No other centrally acting drugs were permitted except lorazepam and benztropine.
Clinical assessments were conducted on days 0, 14, 28, and 42. The psychopathology instrument was the Positive and Negative Syndrome Scale
(9), including the positive, negative, and general psychopathology subscales. Drug safety was evaluated by physical examinations, laboratory tests, the Extrapyramidal Symptom Rating Scale
(10), and the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale
(11). Patients were genotyped for 5-HT
2A polymorphisms by polymerase chain reaction amplification of DNA and digestion with MspI for detection of 102-T/C
(2).
Positive and Negative Syndrome Scale total and subscale scores were used as a measure of response to risperidone. Potential prognostic factors included T/C genotype as well as treatment duration, baseline Positive and Negative Syndrome Scale score, gender, age, education level, diagnosis subtype, age at illness onset, duration of illness, number and total duration of previous hospitalizations, and endpoint risperidone dose. Because there were repeated assessments, multiple linear regression with the generalized estimating equation method was used to adjust the within-subject dependence. Statistical significance was defined as p<0.05.
Results
One hundred patients completed the assessments on day 0 and day 14 (the first posttreatment visit) and were eligible for data analysis. The numbers of patients actively participating on days 28 and 42 were 95 and 81, respectively. The mean age of the 100 patients was 34.0 years (SD=9.7); 55 were men, and 45 were women. The mean age at illness onset was 24.4 years (SD=8.2), the mean illness duration was 114.8 months (SD=91.0), the mean number of previous hospitalizations was 1.9 (SD=2.5), the mean duration of previous hospitalizations was 23.7 weeks (SD=42.7), and the mean level of education was 10.7 years (SD=3.1). The distribution of schizophrenia subtypes was 66 paranoid, 15 disorganized, and 19 undifferentiated. The mean risperidone dose at endpoint was 4.2 mg/day (SD=1.4). The genotype distribution did not deviate significantly from the Hardy-Weinberg equilibrium: 102-T/T in 43 patients, C/C in 10, and T/C in 47. The allele frequency of 102-T was similar to that in the earlier study enrolling Han Chinese (2) but higher than that in Western populations (1, 3).
The mean Positive and Negative Syndrome Scale total scores declined during risperidone treatment: 87.4 (SD=14.1) at baseline and 70.2 (SD=15.0) at endpoint. The mean positive subscale scores declined from 23.2 (SD=4.1) to 17.1 (SD=5.0). The mean negative scores declined from 25.9 (SD=5.4) to 22.6 (SD=5.6), respectively, and the general psychopathology scores declined from 38.5 (SD=8.1) to 30.7 (SD=7.1). However, the distributions of the Positive and Negative Syndrome Scale total and subscale scores were skewed to the right (data not shown) and unsuitable for regression analyses. The value of each Positive and Negative Syndrome Scale total or subscale score was thus transformed to its natural logarithm to obtain normal distributions (data not shown).
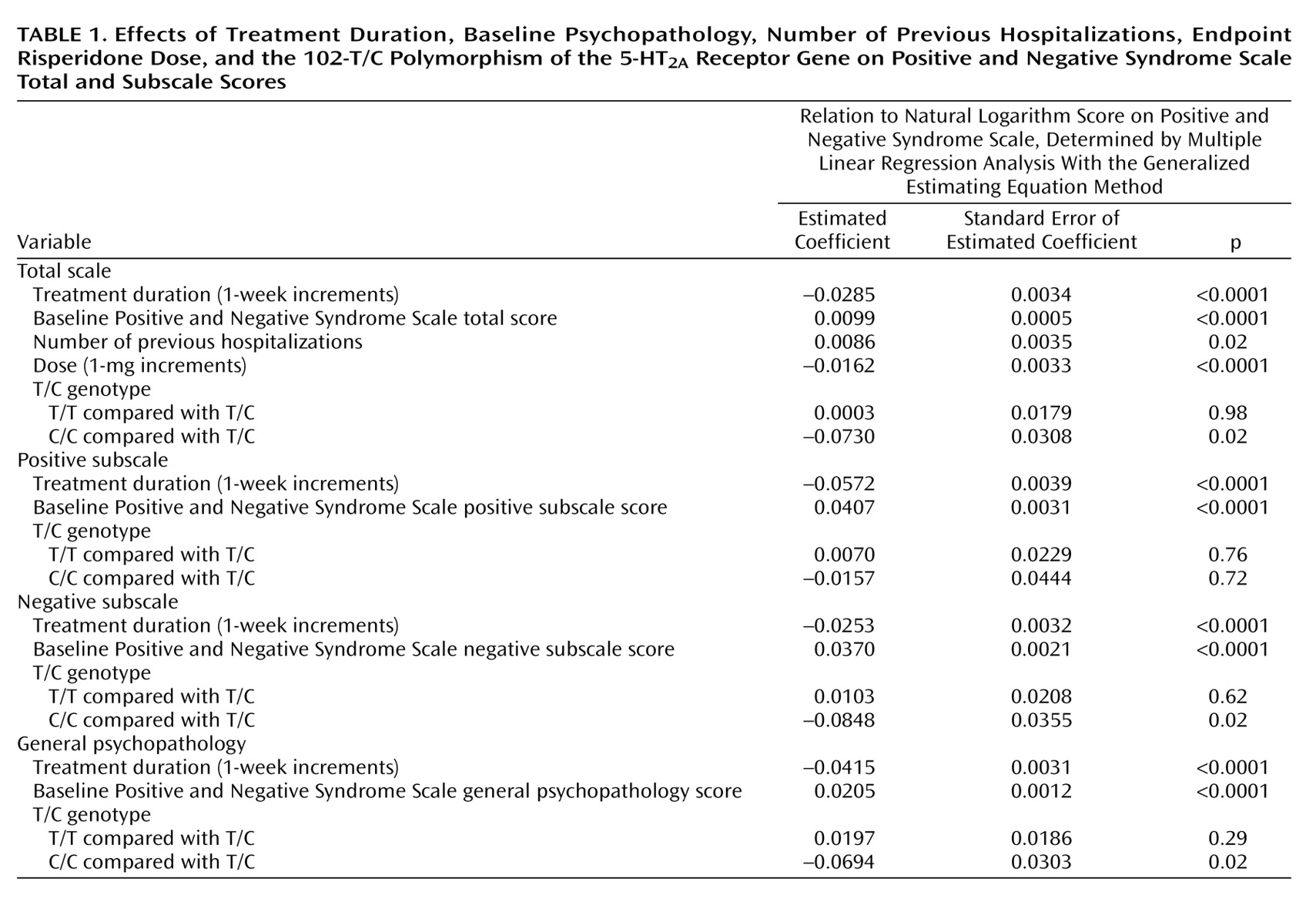
Table 1 shows that, after adjustment for the effects of treatment duration and patient-related variables, the 102-C/C genotype was related to better clinical performance. Compared with patients who had the T/C genotype, the Positive and Negative Syndrome Scale total scores of patients with the C/C genotype were 7.04% lower (e-0.0730=0.9296; 1–0.9296=0.0704), their negative subscale scores were 8.13% lower (e-0.0848=0.9187; 1–0.9187=0.0813), their general psychopathology scores were 6.70% lower (e-0.0694=0.9330; 1–0.9330=0.0670), but their positive subscale scores were not lower. Patients with the T/C and T/T genotypes did not differ significantly in any subscale score or the total score. In addition, each one-time increment in number of previous hospitalizations increased the Positive and Negative Syndrome Scale total score by 0.86% (e-0.0086=1.0086; 1.0086–1=0.0086), and each 1-mg increment in the daily dose of risperidone decreased the Positive and Negative Syndrome Scale total score by 1.61% (e-0.0162=0.9839; 1–0.9839=0.0161). Other potential prognostic factors did not significantly influence clinical performance.
Discussion
Our findings suggest that the 5-HT
2A receptor 102-C/C genotype may predict superior risperidone response (particularly for negative symptoms rather than positive symptoms) in patients with acutely exacerbated schizophrenia. However, it has been shown that the 102-C/C genotype is more frequent among clozapine nonresponders than responders
(3). Several reasons may contribute to this discrepancy. First, the subjects in the clozapine studies were treatment-resistant patients
(3), but those in the present study were acutely ill patients. Second, clozapine is distinguished from risperidone in pharmacological profile
(6). Third, subject ethnicity and study methodology differed between risperidone and clozapine studies.
The preliminary results in this study support the hypothesis that the 5-HT
2A receptor 102-T/C polymorphism or, alternatively, another genetic variation that is in linkage disequilibrium
(3) may influence individual response to risperidone. However, we might have had a chance false positive finding. Further studies in other ethnic populations or in chronically ill patients are warranted. In addition, possible effects of other 5-HT
2A receptor polymorphisms such as 452-His/Tyr
(3–
5) on risperidone efficacy also require investigation.