Extrapyramidal symptoms and limited efficacy are serious limitations of conventional antipsychotics, while high acquisition costs have put the novel antipsychotics beyond the reach of patients in lower-income countries
(1). Omega-3 polyunsaturated fatty acids may offer an affordable treatment alternative. Supportive findings include low levels of omega-3 polyunsaturated fatty acids in red blood cells
(2) and the brain
(3) in schizophrenia. Open-label supplemental studies have suggested a beneficial effect for omega-3 polyunsaturated fatty acids in schizophrenia. A pilot study found that eicosapentaenoic acid (EPA) was superior to docohexaenoic acid and placebo when added to standard antipsychotic treatment
(4), although another double-blind study of EPA versus placebo found no improvement of symptoms with EPA
(5). In a dose-ranging study, ethyl-EPA (E-EPA) doses of 1, 2, and 4 g/day were no better than placebo, although subjects taking clozapine with E-EPA improved significantly, the effect being greatest with a 2 g/day dose of E-EPA
(6). Recent reviews of the treatment of schizophrenia with omega-3 polyunsaturated fatty acids found results to be encouraging but preliminary
(7), somewhat conflicting
(8), and requiring independent replication
(9).
Method
Forty subjects aged 18 to 55 years who met DSM-IV criteria for schizophrenia were recruited. All had received fixed doses of antipsychotics for at least 6 months and had a Positive and Negative Syndrome Scale
(10) total score >50. Exclusion criteria comprised substance abuse and significant medical conditions. The University of Stellenbosch ethics committee approved the study, and subjects provided informed, written consent.
This was a randomized, parallel-group, double-blind, placebo-controlled, add-on study conducted over 12 weeks. Patients were assessed at baseline and at weeks 3, 6, 9, and 12 by means of the Positive and Negative Syndrome Scale and the Extrapyramidal Symptom Rating Scale
(11). Subjects were randomly assigned to receive either 3 g/day of E-EPA supplement in encapsulated form (three 500-mg capsules twice daily) (Laxdale Ltd., Stirling, Scotland) or placebo (3 g/day of medicinal liquid paraffin BP) in addition to the medication that they had been receiving.
The primary outcome measure was the percentage change in Positive and Negative Syndrome Scale total scores between baseline and 12 weeks. Secondary efficacy measures were the changes in the Positive and Negative Syndrome Scale positive, negative, and general psychopathology subscale scores. Extrapyramidal symptoms were assessed by the changes in total Extrapyramidal Symptom Rating Scale scores and in the scale’s subscores for dyskinesia, dystonia, akathisia, and parkinsonism. A dietician reviewed the dietary intake of each subject at baseline and throughout the study. EPA intake was calculated according to standard food supplementation tables provided by the South African Medical Research Council.
Comparisons were performed with Student’s t test. An intent-to-treat design was used, with the last observation carried forward for any subject who did not complete the 12-week study. An additional analysis was performed with analysis of covariance (ANCOVA) to control for the effects of dietary EPA, medication, duration of illness, and gender. A p value of <0.05 was considered statistically significant. All tests were two-tailed.
Results
Baseline demographic and clinical variables were similar for the two groups. The age and illness duration were 46.2 years (SD=10.6) and 23.1 years (SD=8.5) for the E-EPA group, and 43.6 years (SD=13.9) and 22.2 years (SD=12.4) for the placebo group. Antipsychotic doses (chlorpromazine equivalents) were 1011 mg/day (SD=532) for the E-EPA group and 931 mg/day (SD=652) for the placebo group. No additional medication was prescribed throughout the trial, except for occasional analgesics for headache and lorazepam for insomnia. There were no differences between groups regarding dietary intake of omega-3 polyunsaturated fatty acids. All subjects received a balanced diet before and throughout the trial. Dietary EPA intake was generally low, ranging from 0.56 g/week to 1.13 g/week. Antipsychotic medication remained unchanged for all subjects throughout the trial. Nine subjects in each group were receiving clozapine, and the rest were receiving conventional antipsychotics. One subject from the E-EPA group was withdrawn from the trial after taking an overdose of medication. No other serious adverse events were recorded.
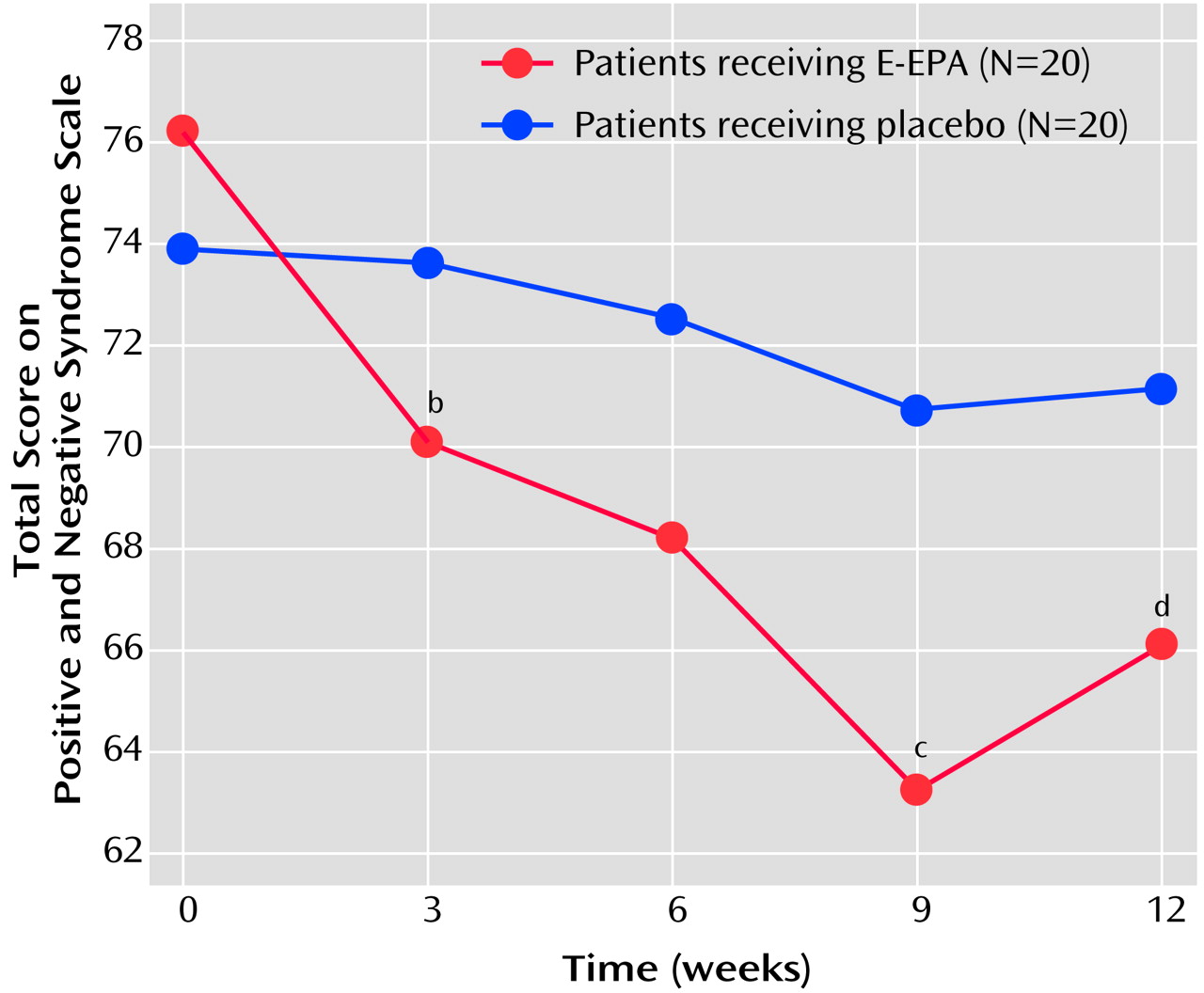
For the primary outcome measure the difference between the groups was statistically significant in favor of the E-EPA group (mean=12.6 [SD=14.0] versus 3.1 [SD=13.3]) (t=2.20, df=38, p=0.03), and this difference remained significant after controlling for effects of dietary EPA, medication, duration of illness, and gender (
Figure 1). The reduction tended to be greater in the E-EPA patients taking conventional antipsychotics than in those taking clozapine (mean=17.4 [SD=12.1] versus mean=6.8 [SD=14.6]) (t=1.80, df=18, p=0.09). For the Positive and Negative Syndrome Scale subscales, the only significant difference was in favor of E-EPA in the percentage change in the general psychopathology subscale score at endpoint (t=2.08, df=38, p=0.04). There were no group differences for the changes in Extrapyramidal Symptom Rating Scale parkinsonism, dystonia, or akathisia scores. However, the E-EPA group showed a significantly greater reduction in Extrapyramidal Symptom Rating Scale dyskinesia scores at 12 weeks (t=2.82, df=38, p=0.008). A further analysis of covariance using change in Positive and Negative Syndrome Scale total score as the dependent variable and change in dyskinesia score as a covariate was performed. The difference between the E-EPA and placebo groups was no longer significant (F=3.08, df=1, 39, p=0.09), suggesting that reduction in Positive and Negative Syndrome Scale scores is associated with reduction in dyskinesia scores.
Discussion
This study shows a significant advantage for E-EPA over placebo in the primary outcome measure. The between-group difference had reached significance after 3 weeks of treatment, signifying an early onset of action. We regard these results as remarkable, considering the refractory nature of schizophrenia in the subjects. The reduction in dyskinesia scores for the E-EPA group was unanticipated, although an inverse relationship between tardive dyskinesia scores and blood levels of EPA has been reported
(12). Given the chronic nature of the disorder in the subjects, most of these dyskinetic symptoms are likely to have been due to neuroleptic-induced tardive dyskinesia. The following factors limit the generalization of our findings. First, the study was conducted in a small group of subjects. Second, whether E-EPA is an effective antipsychotic on its own is not known. And third, the results of the analysis of covariance suggest that reduction in Positive and Negative Syndrome Scale scores may at least in part be related to reduction in dyskinesia scores.
E-EPA may be an effective, safe, and well-tolerated add-on treatment in chronic schizophrenia. The extent of its antipsychotic activity remains to be determined. The beneficial effect observed on dyskinesia also requires further exploration. If efficacy in psychosis and tardive dyskinesia is confirmed, it is likely to lead to revision of our understanding of the pathophysiology and treatment of these disorders.