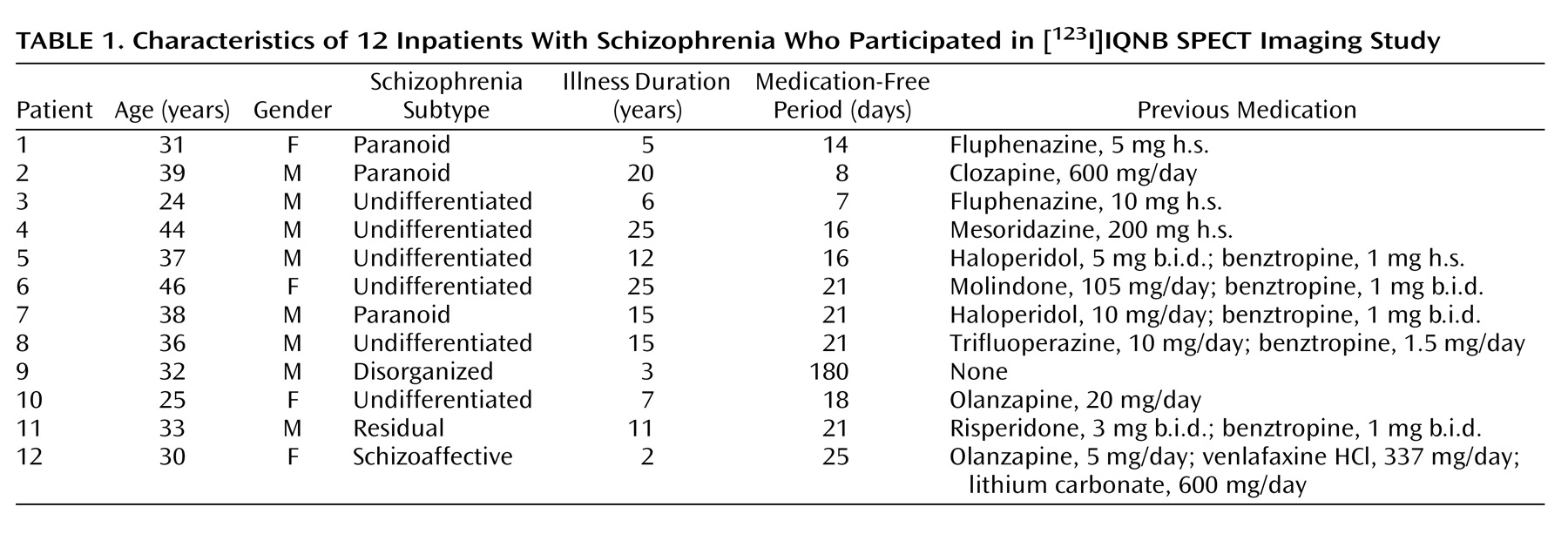
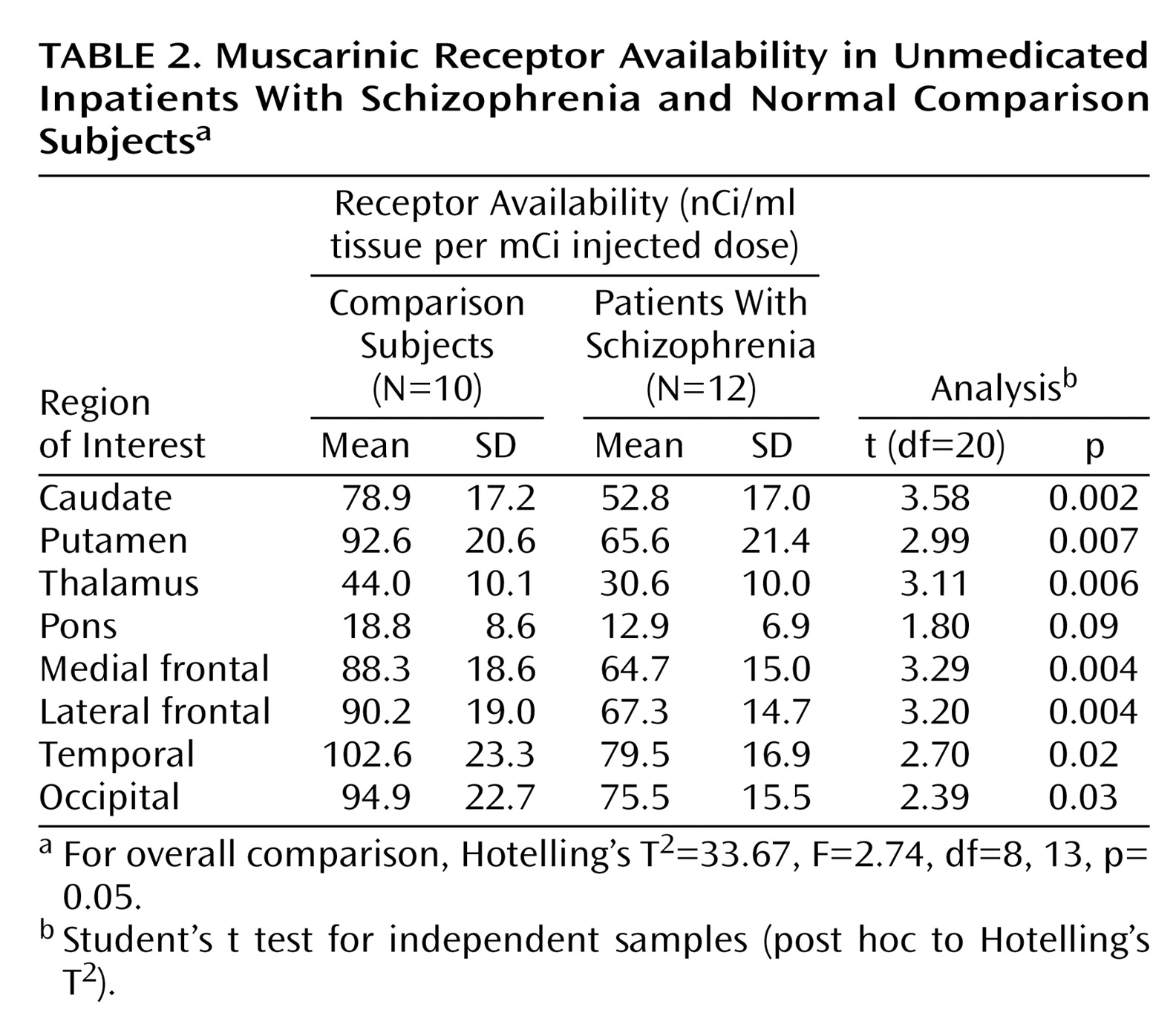
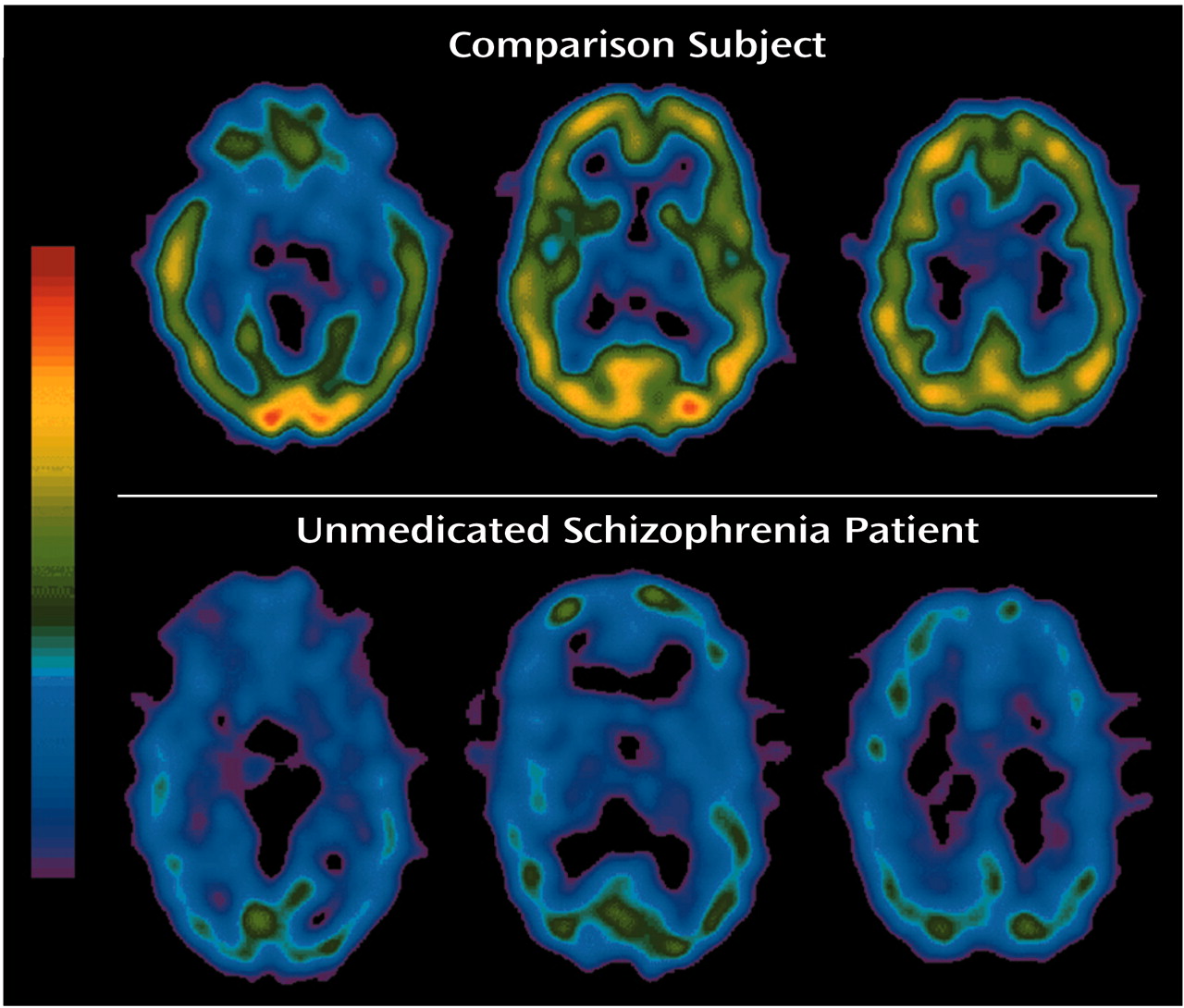
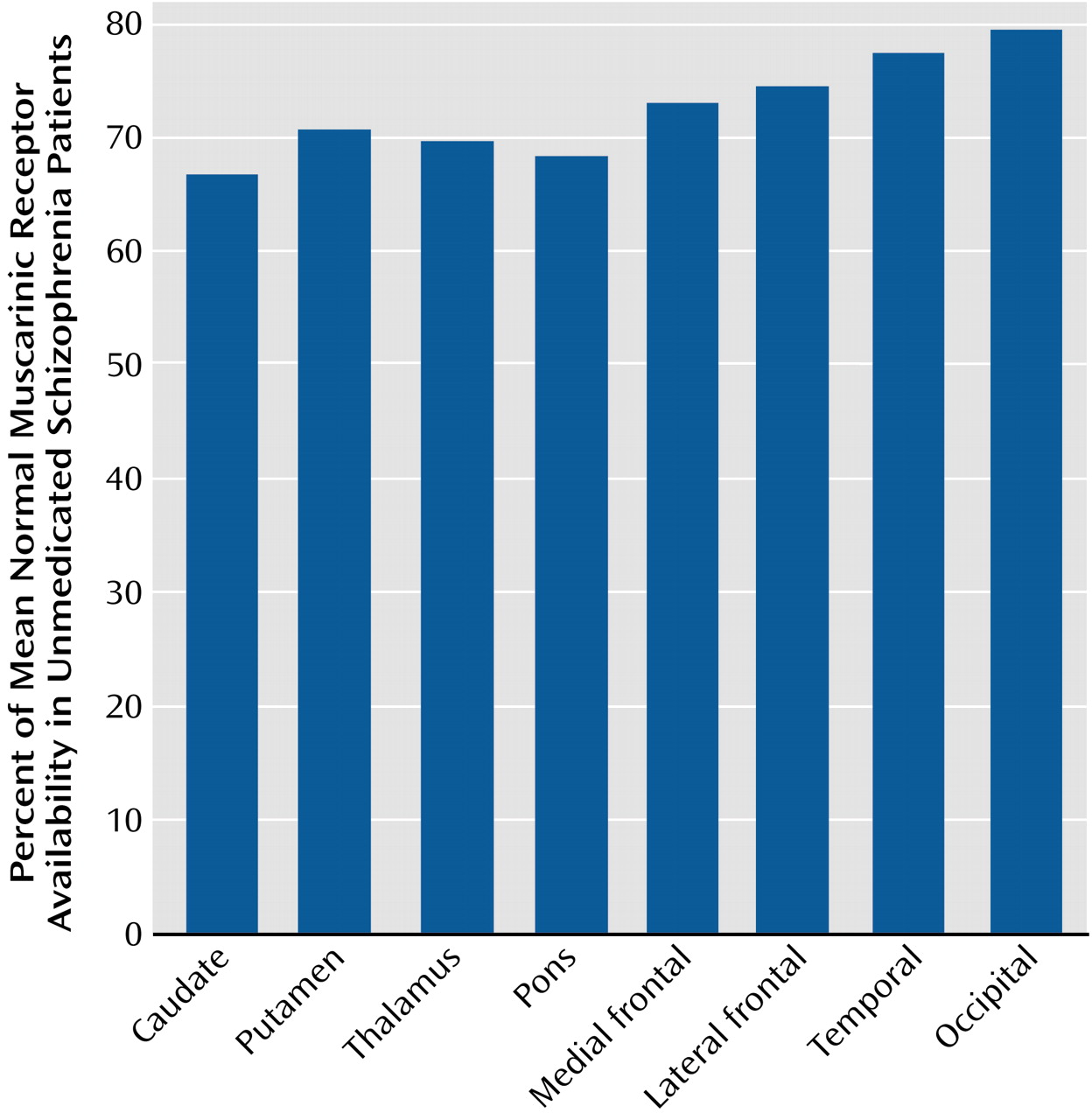
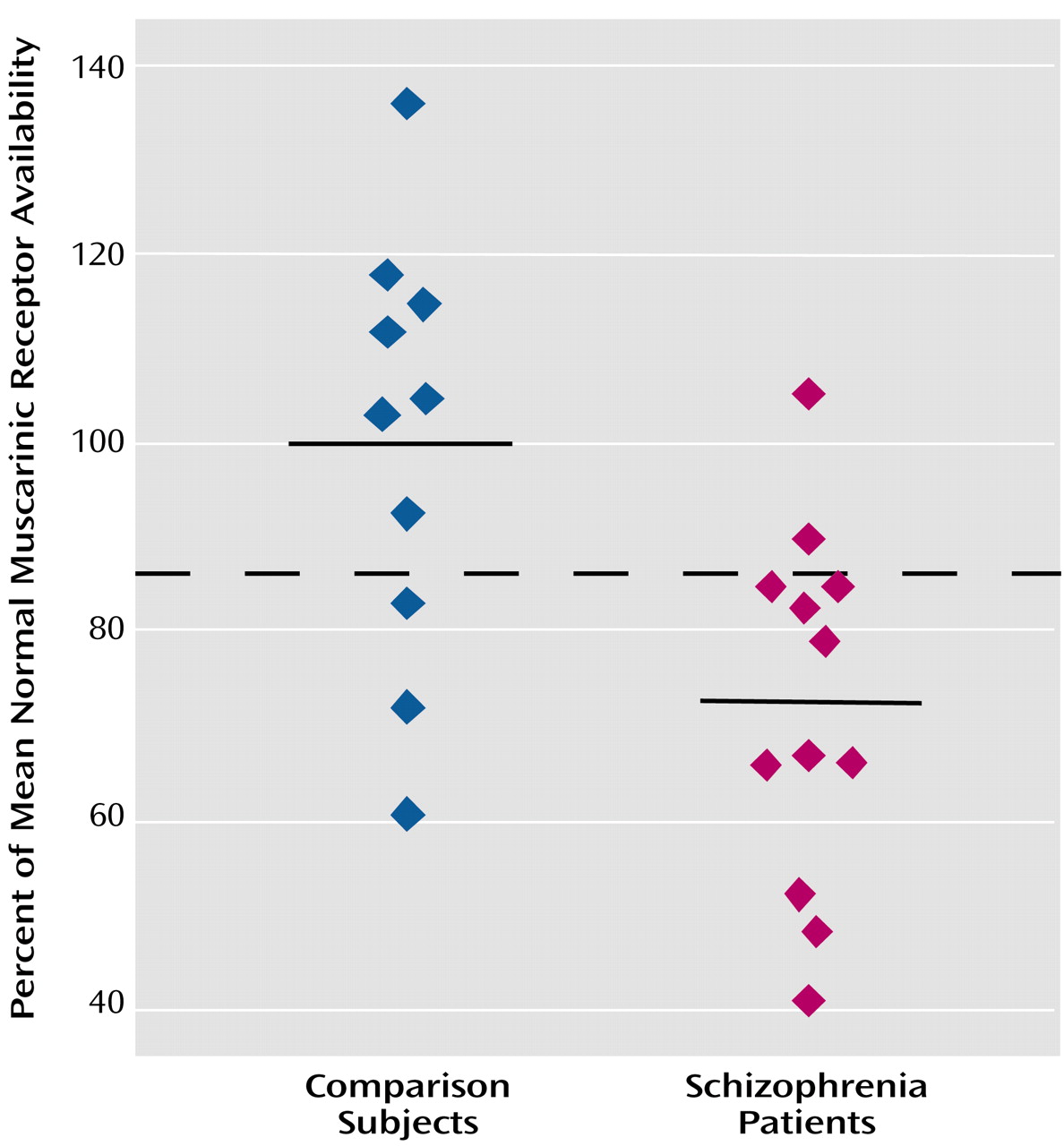
To our knowledge, this is the first study to compare in vivo muscarinic receptor availability between unmedicated patients with schizophrenia and age- and gender-matched normal subjects. We found that the muscarinic receptor availability in patients was significantly less (20%–33% less) than that of normal subjects in the cortex, basal ganglia, and thalamus.
The Muscarinic Cholinergic System in Schizophrenia
Our data are consistent with diverse evidence from earlier pharmacological, clinical, and endocrinological observations that implicated the muscarinic cholinergic system in schizophrenia. Several clinical studies have looked at the treatment with anticholinergic agents of patients with schizophrenia. In unmedicated patients with schizophrenia, biperiden, a commonly used anticholinergic agent, led to significant decreases in negative symptoms as well as significant increases in positive symptoms
(2,
3). These findings were interpreted as reflecting greater cholinergic activity in schizophrenia. Similar findings of a worsening effect of anticholinergics on positive symptoms were also reported in patients with schizophrenia who were medicated with antipsychotics
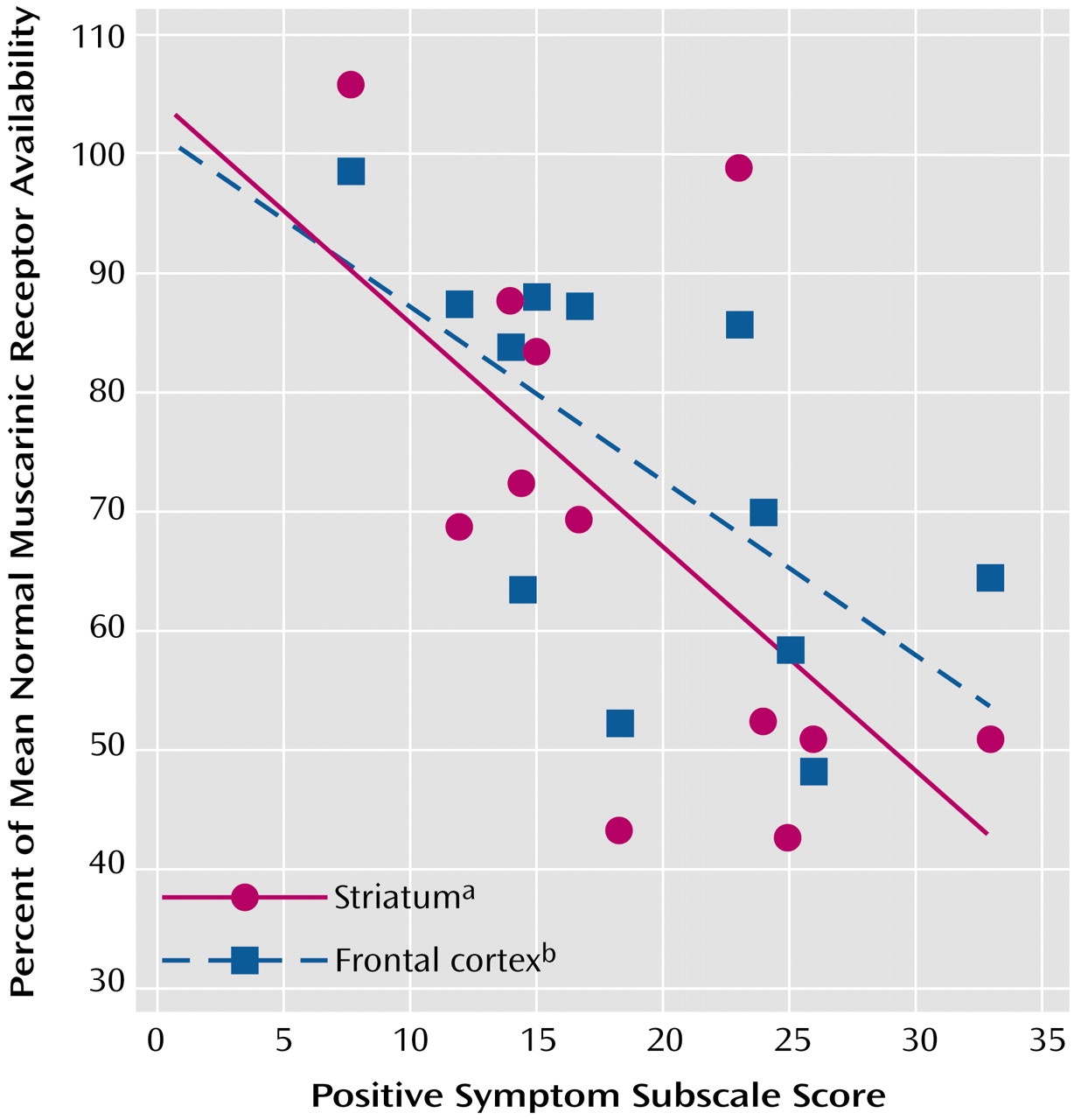
(23). These earlier findings are compatible with our observation of a negative correlation between muscarinic receptor availability and positive symptoms because treatment with anticholinergics should further reduce muscarinic receptor availability.
In addition to these behavioral observations, sleep studies and endocrinological challenge studies suggest an alteration of the muscarinic system in schizophrenia. In sleep studies, patients with schizophrenia show reduced REM latency, which has been linked to “muscarinic supersensitivity”
(4,
5). Endocrinological challenge studies in patients with schizophrenia have reported a greater growth hormone response to pyridostigmine, a cholinesterase inhibitor, suggestive of greater cholinergic tone
(6). Anticholinergic treatment may even have an effect on mortality: the lack of treatment with anticholinergics has been associated with reduced survival in elderly patients with schizophrenia
(24).
Our finding of reduced muscarinic receptor availability is compatible with the results of neuropathological studies that have examined the muscarinic system in schizophrenia. Analyzing the receptor availability for different neurotransmitters in the frontal cortex in two samples of patients with schizophrenia and normal comparison subjects, Bennett et al.
(7) found significantly lower
3H-QNB binding in patients with schizophrenia. Dean et al.
(8) reported a significant reduction of M
1 receptor density in the caudate-putamen of patients with schizophrenia compared with normal subjects. Although patients with schizophrenia previously treated with anticholinergics had even lower binding, the M
1 receptor density was also significantly reduced in subjects who had not received anticholinergics. A similar reduction was also reported for the M
2 and M
4 subtypes of the muscarinic receptors in the caudate and putamen
(9). In other studies from the same group, M
1 and M
4 receptor density was reduced in patients with schizophrenia in regions of the hippocampal formation
(10) and in the prefrontal cortex
(11). The results of these studies contrast with two other studies that found greater muscarinic receptor density in patients with schizophrenia in the orbitofrontal and medial frontal cortex
(25) as well as the putamen
(26). Another study counted cholinergic neurons in the pedunculopontine nucleus and reported significantly more in patients with schizophrenia than in normal comparison subjects but no difference between groups in noradrenergic neurons in the locus ceruleus
(27).
Several enzymes involved in the regulation of acetylcholine metabolism also have been investigated. Concentrations of choline acetyltransferase, the enzyme-synthesizing acetylcholine, were significantly smaller in the pons of patients with schizophrenia than normal comparison subjects, but there were no differences between these groups in other brain regions
(28). In contrast to this finding, greater levels of choline acetyltransferase in the hippocampus, caudate, putamen, and nucleus accumbens in patients with schizophrenia have also been described
(29). In another study
(30), cholinergic markers (choline acetyltransferase and acetylcholinesterase) were diminished in the brains of subjects with Alzheimer’s disease but not different in the brains of patients with schizophrenia compared with normal subjects. Although the reasons for these inconsistencies in the postmortem literature are unclear, the possibilities of uncontrolled effects of chronic illness and treatment are difficult to address in such studies.
It is of interest to speculate on the relationship of the cholinergic system to other neurotransmitter systems implicated in schizophrenia. The dopaminergic and cholinergic systems in the brain interact directly and indirectly in the striatum and in the cortex. Functional muscarinic receptors have been shown to exist on dopaminergic neurons in single unit recordings
(31) as well as in microdialysis experiments
(32). In the substantia nigra, cholinergic fibers have synaptic contact with dopaminergic neurons
(33). Similarly, it has been suggested that ventral tegmental area dopamine cells have functional muscarinic receptors and that the activation of these receptors stimulates the release of dopamine
(34). In synaptosomes, acetylcholine potentiates the release of dopamine. This effect can be counteracted by atropine
(35). In a positron emission tomography (PET) study of human volunteers, the application of muscarinic cholinergic antagonists resulted in increased striatal dopamine release
(36). The activation of dopaminergic cells in the midbrain by muscarinic agonists involves M
1-like receptors
(37). The effects of the application of muscarinic agonists on dopaminergic neurons depends on the pattern of activation and ranges from hyperpolarization after brief activation of muscarinic receptors to desensitization after prolonged activation
(38). Acetylcholine itself exerts very little effect on dopaminergic neurons, but muscarinic and nicotinic agonists increased their firing rate
(31). On the basis of these observations, Yeomans
(39) has speculated that schizophrenia may be caused by an overactivation of cholinergic neurons in the pedunculopontine and the laterodorsal tegmental nucleus, resulting in activation of dopaminergic neurons. Such speculation would suggest that our findings represent a secondary down-regulation of postsynaptic cholinergic receptors. However, it is difficult to arrive at a simple scheme to explain the basic pharmacological data implicating an overactive cholinergic system, evidence that anticholinergics increase positive symptoms in patients, and the results in schizophrenic brain tissue (both the postmortem and our in vivo data).
Other neurotransmitter systems have been studied in vivo in schizophrenia. Most of these studies failed to find significant differences in baseline receptor availability between unmedicated patients with schizophrenia and normal comparison subjects. Although one study found an increase in dopamine D
2 receptor availability in medication-free patients with schizophrenia
(40), subsequent studies failed to replicate significant differences in dopamine D
2 receptor availability
(41–
43). Similarly, no significant changes have been found for the in vivo availability of the dopamine transporter
(44,
45). However, unmedicated patients with schizophrenia showed significantly higher dopamine release after an amphetamine challenge than normal comparison subjects, suggesting that the dopaminergic neurons may be more responsive in schizophrenia
(46–
48).
Methodological Considerations
In the interpretation of our data, the potential role of previous medication treatment needs to be taken into consideration. Although all subjects had been medication free for at least 7 days before the SPECT scan (and most subjects considerably longer), they had taken antipsychotics before this period. Some subjects had also taken anticholinergics or antipsychotics with known anticholinergic properties. Although little is known about the effects of antipsychotic and antimuscarinic treatment on muscarinic cholinergic receptors in humans, this issue has been studied in animals
(49–
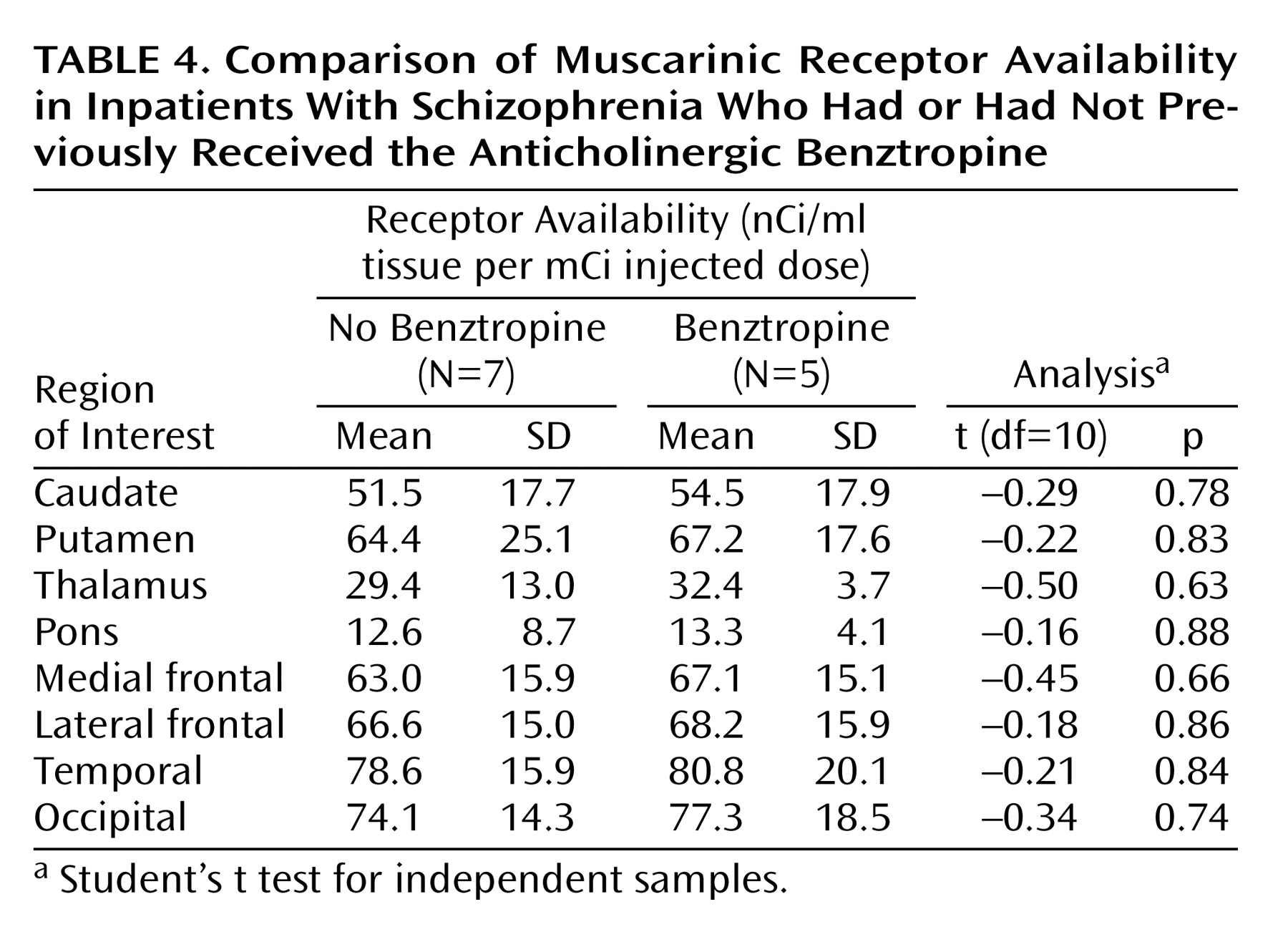
58). The results have been inconsistent, ranging from a decrease in muscarinic receptor density to no change to an increase. However, most of the data suggest an up-regulation of muscarinic receptors after pharmacological treatment with antagonists, in contrast to our results. Carryover effects from previous pharmacological treatments seem unlikely because muscarinic receptor availability did not correlate with the duration of the medication-free period. Reduced muscarinic receptor availability was found in all subjects regardless of their previous pharmacological treatment (typical versus atypical antipsychotics, use of anticholinergics versus no anticholinergics, and antipsychotics with anticholinergic properties versus no anticholinergic properties).
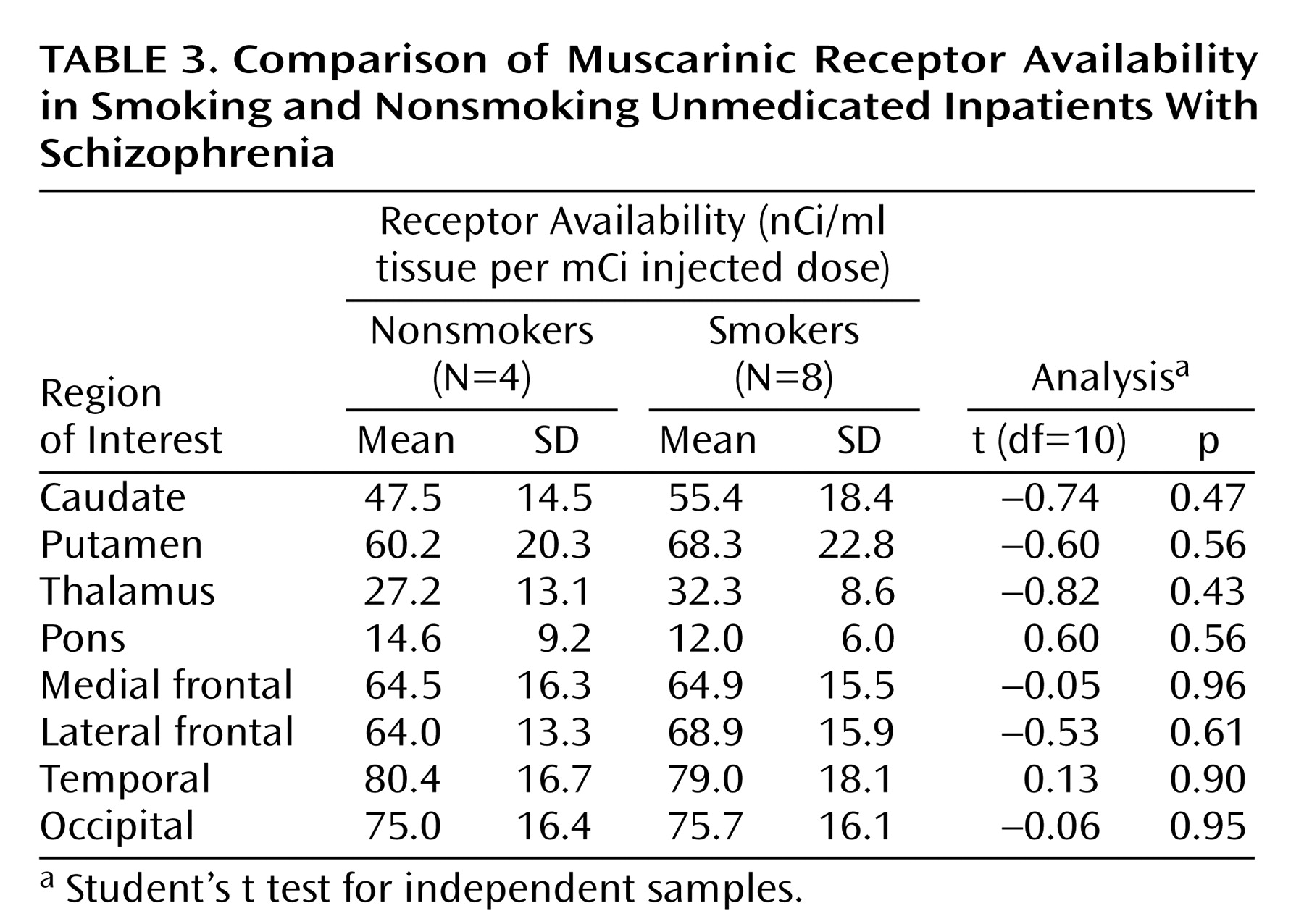
In clinical practice it is widely recognized that the prevalence of smoking among patients with schizophrenia far exceeds the prevalence of smoking in the general population. Consistent with this observation, smokers are overrepresented among our small group of patients with schizophrenia. However, when comparing muscarinic receptor availability among smokers and nonsmokers in the patient sample, we found even lower muscarinic receptor availability among the nonsmoking schizophrenic subjects. The small number of subjects does not allow firm conclusions, but these results suggest that smoking per se is not responsible for the low muscarinic receptor availabilities found in our patients.
Similarly, it seems unlikely that age-related effects could account for our observations. Our group of comparison subjects did not differ significantly in mean age from the group of patients, and muscarinic receptor availability did not significantly correlate with age in either group. This observation of no significant decline with age in muscarinic receptor availability is consistent with our findings of no significant decline with age in a larger group of 20 healthy comparison subjects ranging in age from 23 to 75 years
(59).
Other potential confounds that might lead to erroneously low SPECT region of interest measurements are structural differences in the brain tissue between groups. However, our a priori examination of the MRI scan for each individual in both groups revealed no gross structural differences that might account for the reductions we have observed. Although subtle differences in brain structure or tissue composition might account partially for our findings, it should be noted that the reductions in muscarinic receptor availability that we have determined in vivo are quantitatively in good agreement with corresponding in vitro studies of postmortem tissue
(7–
11). Thus, we feel it is unlikely that structural differences had a significant effect on our measurements.
SPECT and PET imaging of neurotransmitter receptors uses specific tracers labeled with radioactivity. In our previous [
123I]IQNB SPECT studies in patients with schizophrenia treated with olanzapine or clozapine
(16), we were able to show that [
123I]IQNB SPECT is sensitive to receptor occupancy by medication. Reduced receptor availability in unmedicated subjects can be attributable to either reduced numbers of receptors or increased occupancy by endogenous neurotransmitter, in this case acetylcholine. Although a hypercholinergic state cannot be excluded on the basis of our SPECT study, the neuropathological reports of reduced muscarinic receptor density suggest that our findings reflect a reduction in receptor density. The two effects, however, are not mutually exclusive, and a hypercholinergic state would be expected to be associated with a corresponding down-regulation of muscarinic receptors (see discussion above).
Muscarinic Receptor Selectivity and Treatment Implications
[
123I]IQNB binds very selectively and with high affinity to muscarinic receptors
(12). [
123I]IQNB does not allow discrimination between the different subtypes of the muscarinic receptors. So far, five genetically distinct subtypes of the muscarinic receptor (M
1, M
2, M
3, M
4, and M
5) are known to be expressed in the brain with different anatomical distributions. One of the unexpected findings in our study is the relatively widespread and regionally nonspecific pattern of reduction in muscarinic binding. This observation suggests that the availability of multiple muscarinic receptor subtypes is reduced in schizophrenia. This is also compatible with the results of neuropathological studies, which have shown decreases in schizophrenia in the different muscarinic receptor subtypes (e.g., M
1 and M
2/M
4). The genes for the different subtypes of the muscarinic receptor subtypes are located on different chromosomes. Although a common transcription factor for all muscarinic receptor genes might explain the reduction in the density of different muscarinic receptor subtypes, little is known about the regulation of the expression of the muscarinic receptor genes, and there is no evidence that they share a common transcription factor
(60,
61).
The role of the muscarinic system in schizophrenia recently has been evaluated as a potential novel pharmacological approach for the treatment of psychosis. (5R,6R)6-(3-Propylthio-1,2,5-thiadiazol-4-yl)-1-azabicyclo[3.2.1]octane (PTAC) is a muscarinic receptor ligand with partial agonist effects at muscarinic M
2 and M
4 receptors and antagonist effects at M
1, M
3, and M
5 receptors. PTAC selectively inhibits dopamine cell firing as well as the number of spontaneously active dopamine cells. Since this substance proved to have functional dopamine receptor antagonistic properties in animals, it may be further investigated as a novel approach for the treatment of schizophrenia
(62). Treatment with other muscarinic agonists, including xanomeline, resulted in behavioral responses similar to treatment with traditional antipsychotics in animal models that may be applicable to humans
(63).
In conclusion, there is mounting evidence that the muscarinic cholinergic system is altered in schizophrenia. Our study shows a significant reduction of muscarinic receptor availability in unmedicated schizophrenic subjects. We cannot address whether this finding represents a primary pathophysiological phenomenon or a secondary effect of other factors. These results need to be replicated in additional groups of patients, especially those without previous medication exposure and without chronic illness. The availability of a noninvasive neuroimaging method for measurement of muscarinic binding in vivo makes this possible. These latter replication studies might apply the retrospective “diagnostic” criteria that we found useful in this study to test their utility as a diagnostic aid in a prospective manner.