Borderline personality disorder is marked by notable reactivity of mood and impulsive aggression. Because response to antidepressants and mood stabilizers has typically been clinically modest in this patient group
(1), the identification of novel treatments is needed. Candidates include omega-3 fatty acids, such as eicosapentaenoic acid and docosahexaenoic acid, which are commonly found in seafood and have beneficial effects and none of the adverse side effects commonly associated with pharmacotherapy. In cross-national studies, greater seafood consumption was associated with lower rates of bipolar disorder (30-fold range) and major depression (50-fold range)
(2). In placebo-controlled trials, a mixture of these fatty acids was found to be an effective adjunctive agent for patients suffering from bipolar disorder
(3), and ethyl-eicosapentaenoic acid (E-EPA) was found to have a beneficial adjunctive effect for patients suffering from recurrent depression
(4). Because of the shared symptoms of borderline personality disorder and these mood disorders, a double-blind, placebo-controlled trial of E-EPA seemed warranted.
Method
Recruitment of women between the ages of 18 and 40 was accomplished through advertisements in Boston newspapers. These ads asked, “Are you extremely moody? Do you often feel out of control? Are your relationships painful and difficult?” Subjects were initially screened by telephone to assess whether they met DSM-IV criteria for borderline personality disorder by using the borderline module of the Diagnostic Interview for DSM-IV Personality Disorders
(5). A general medical and psychiatric history was also taken at the time of first telephone contact. Potential subjects were excluded if they were medically ill, were currently being prescribed any psychotropic medication, were taking E-EPA supplements or ate more than 1–2 servings of fatty fish per week, were actively abusing alcohol or drugs, or were acutely suicidal.
Subjects were next invited to participate in face-to-face interviews. At that time, the study procedures were fully explained, and written informed consent was obtained. Two semistructured diagnostic interviews were then administered to each subject: the Structured Clinical Interview for DSM-IV Axis I Disorders
(6) and the Revised Diagnostic Interview for Borderlines (DIB-R)
(7). Two observer-rated scales were also administered: the Modified Overt Aggression Scale
(8) and the Montgomery-Åsberg Depression Rating Scale
(9).
Subjects were included if they met both DIB-R and DSM-IV criteria for borderline personality disorder. They were excluded if they met current or lifetime criteria for schizophrenia, schizoaffective disorder, or bipolar I or bipolar II disorder or were currently in the midst of a major depressive episode.
Study duration was 8 weeks. Subjects were seen every week for the first month and then biweekly for the next month. Both psychiatric rating scales were readministered at each subsequent visit. Side effects were also assessed at these visits with a structured questionnaire.
Subjects received two capsules per day (beginning the day after their baseline assessment); each capsule contained either 500 mg of 97% E-EPA or a placebo (mineral oil). One gram was chosen as the dose most likely to be effective on the basis of unpublished studies in depression (David Horrobin, personal communication, Feb. 1, 2001). Capsules were supplied by Laxdale Pharmaceuticals (Stirling, U.K.).
Between-group baseline demographic variables and clinical history variables were analyzed by using chi-square analyses for categorical variables and Student’s t test for continuous variables. Student’s t test was also used to analyze the between-group difference on the baseline value of the mean Montgomery-Åsberg Depression Rating Scale score (which was normally distributed). The nonparametric Wilcoxon rank sum test was used to analyze the between-group difference on the mean Modified Overt Aggression Scale score because of the skewed distribution of this variable.
Random effects regression modeling methods were used to assess between-group differences on both outcome measures using all available panel data. Baseline value (for each subject), treatment status, and time were the independent variables in these modeling analyses of the study’s two primary outcome measures.
Results
Thirty subjects were randomly assigned to E-EPA (N=20) or placebo (N=10). This 2:1 randomization ratio was chosen for this pilot study to allow us to gain more experience working with eicosapentaenoic acid. No significant differences were found at baseline on either demographic characteristics or treatment histories. The mean age of the 30 women in this study was 26.3 years (SD=6.2), 23 (76.7%) were white, and on average, they were functioning in the lower half of the fair range of the Global Assessment of Functioning Scale (mean=54.5, SD=7.7). In terms of prior psychiatric treatment, 25 (83.3%) had been in psychotherapy, seven (23.3%) had taken psychotropic medications, and three (10.0%) had been hospitalized for psychiatric reasons.
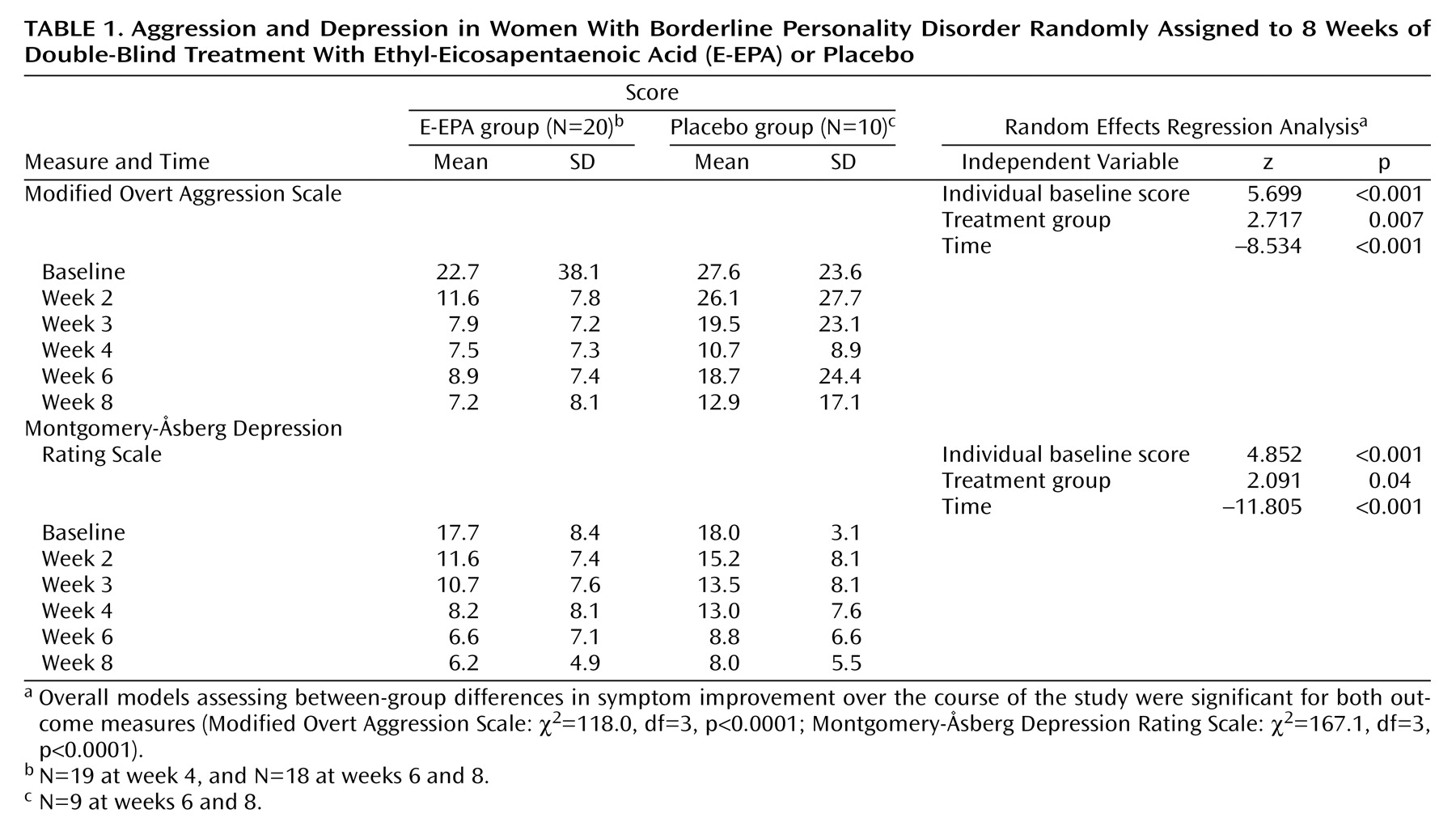
Table 1 summarizes E-EPA versus placebo scores over the course of the entire study for both primary outcome measures. No significant between-group differences in baseline values were found. However, the E-EPA group experienced a significantly greater decrease in their mean Modified Overt Aggression Scale and Montgomery-Åsberg Depression Rating Scale scores than those in the placebo-treated group.
It is important to note that 90.0% of both the E-EPA-treated and placebo-treated subjects remained in the study through week 8. The three subjects who discontinued their participation (two taking E-EPA and one taking placebo) did so because of life events unrelated to the study.
No clinically relevant side effects were elicited. In terms of adverse events, no subject made a suicide gesture or attempt during the course of the study, but 10% of those in each treatment group engaged in one instance of mild self-harm (wrist scratching).
Discussion
The results of this study suggest that E-EPA is a nutriceutical agent that is both well tolerated and may be efficacious for the treatment of moderately disturbed women with borderline personality disorder. Ninety percent of those taking this compound were able to complete the entire 8-week trial and reported no clinically relevant side effects. Those treated with this compound also experienced a significantly greater reduction in their overall aggression as well as their depressive symptoms than those treated with placebo. These results are consistent with the findings of recent reports concerning omega-3 fatty acids as an effective adjunctive treatment for bipolar disorder
(3) and recurrent depression
(4). However, the results of the current study extend these findings by suggesting that omega-3 fatty acids may be an effective form of monotherapy for women with moderately severe cases of borderline personality disorder.
The main limitations of this study are that only women were studied and all subjects were moderately ill. Whether similar results would be found for male subjects or subjects with a more severe symptom picture is unknown.
Studies assessing different doses of E-EPA for longer periods of time in larger samples are needed.