The relationship between dysfunctional attitudes and neurochemical pathology in patients with major depressive episodes and/or chronic self-harm behavior is unclear. However, available evidence suggests that a subpopulation of patients with major depression and/or chronic self-harm behavior have low levels of serotonin (5-HT) stimulation of 5-HT
2 receptors. Investigations using animal models have reported that a chronic lack of stimulation by 5-HT produces an up-regulation of 5-HT
2 receptors in the cortex
(5,
6). Other studies have reported higher than normal levels of 5-HT
2 receptor density in Brodmann’s area 9 in the prefrontal cortex in suicide victims as well as in suicide victims with major depressive episodes
(7–
13). Other indirect measures suggest that the level of 5-HT in the brain is low during major depressive episodes and/or suicidal states. For example, when brain 5-HT levels are low, cerebrospinal fluid concentration of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) is low
(14), and low cerebrospinal fluid 5-HIAA concentration has been found in suicidal subjects as well as in subjects with major depressive episodes
(15,
16). Given these findings, it appears that low 5-HT stimulation of 5-HT
2 receptors in the prefrontal cortex occurs in some patients with major depressive episodes and/or chronic self-harm. Since a higher level of dysfunctional attitudes is an important common symptom in these illnesses, low 5-HT stimulation of 5-HT
2 receptors in the prefrontal cortex may be related to higher levels of dysfunctional attitudes.
Results
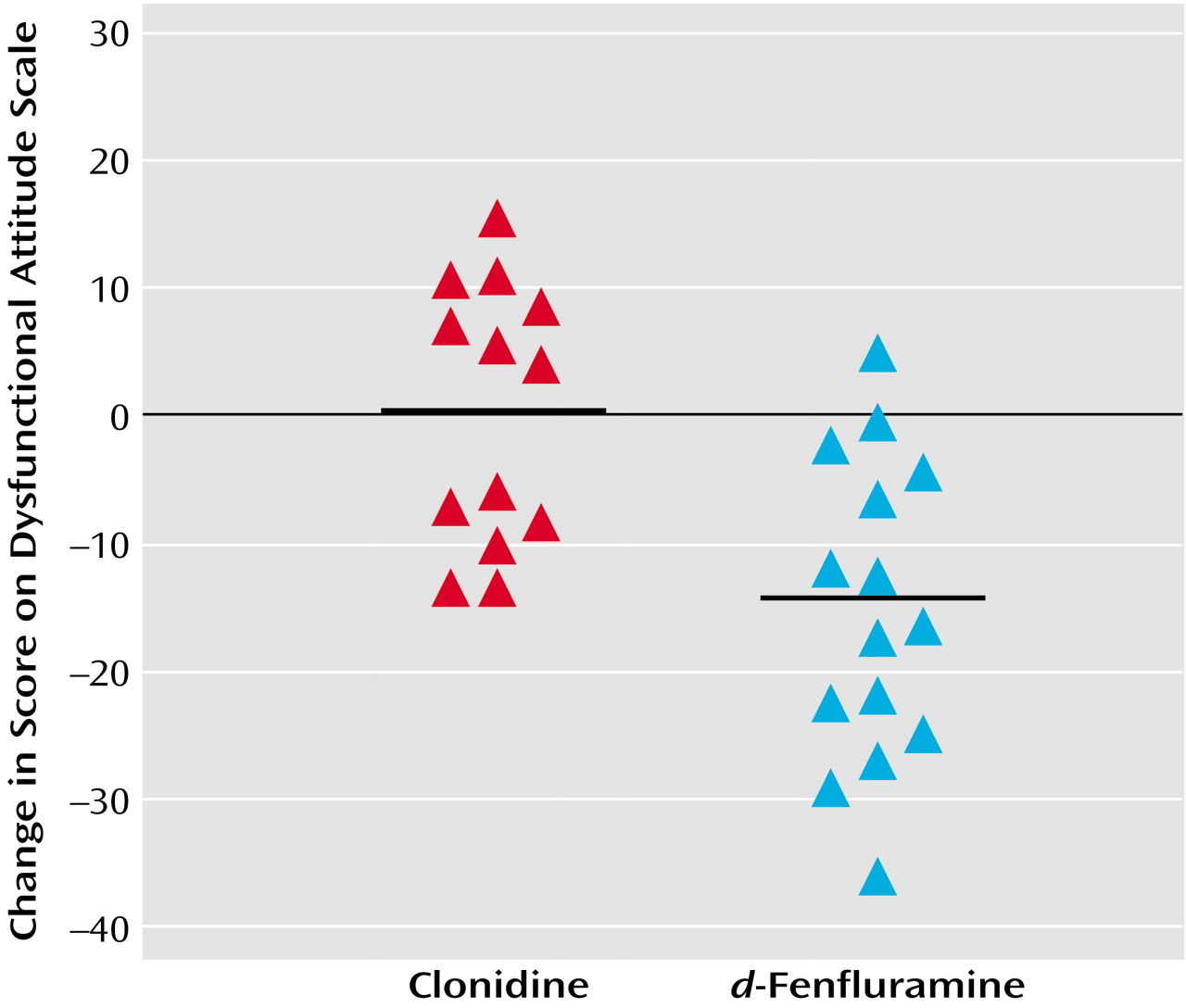
In experiment 1, the decrease in dysfunctional attitudes after
d-fenfluramine was significantly greater than changes observed after clonidine (ANOVA examining effects of drug type and order of administration of the attitude scale versions on change in dysfunctional attitudes—effect of drug type: F=17.3, df=1, 25, p<0.001; effect of order [version A versus version B]: F=15.8, df=1, 25, p=0.001). After the order effect was controlled, the mean decrease in the Dysfunctional Attitude Scale score was 14 points (SD=11) after
d-fenfluramine and 0 points (SD=10) after clonidine. The order effect was controlled by determining the mean change in dysfunctional attitude scores for the subjects who received version A then B of the Dysfunctional Attitude Scale and for the subjects who received version B then A. The difference between the two mean changes in score was determined, and the change in score for each subject was increased or reduced by half the difference between the mean changes in score for the two order groups so that there was no difference between the scores for the two order groups after the transformation.
Figure 1 illustrates the changes in Dysfunctional Attitude Scale scores after
d-fenfluramine or clonidine administration.
No significant changes in the visual analogue scales for mood, anxiety, and energy levels were found (ANOVA examining effects of order of administration of attitude scale versions and drug type—effect of drug type on mood change: F<0.1, df=1, 25, p>0.9; effect of drug type on anxiety change: F<0.1, df=1, 25, p>0.90; effect of drug type on energy level change: F=1.6, df=1, 25, p=0.19). The effects of drug type alone (without considering order of administration of attitude scale versions) on changes in visual analogue scales were also nonsignificant.
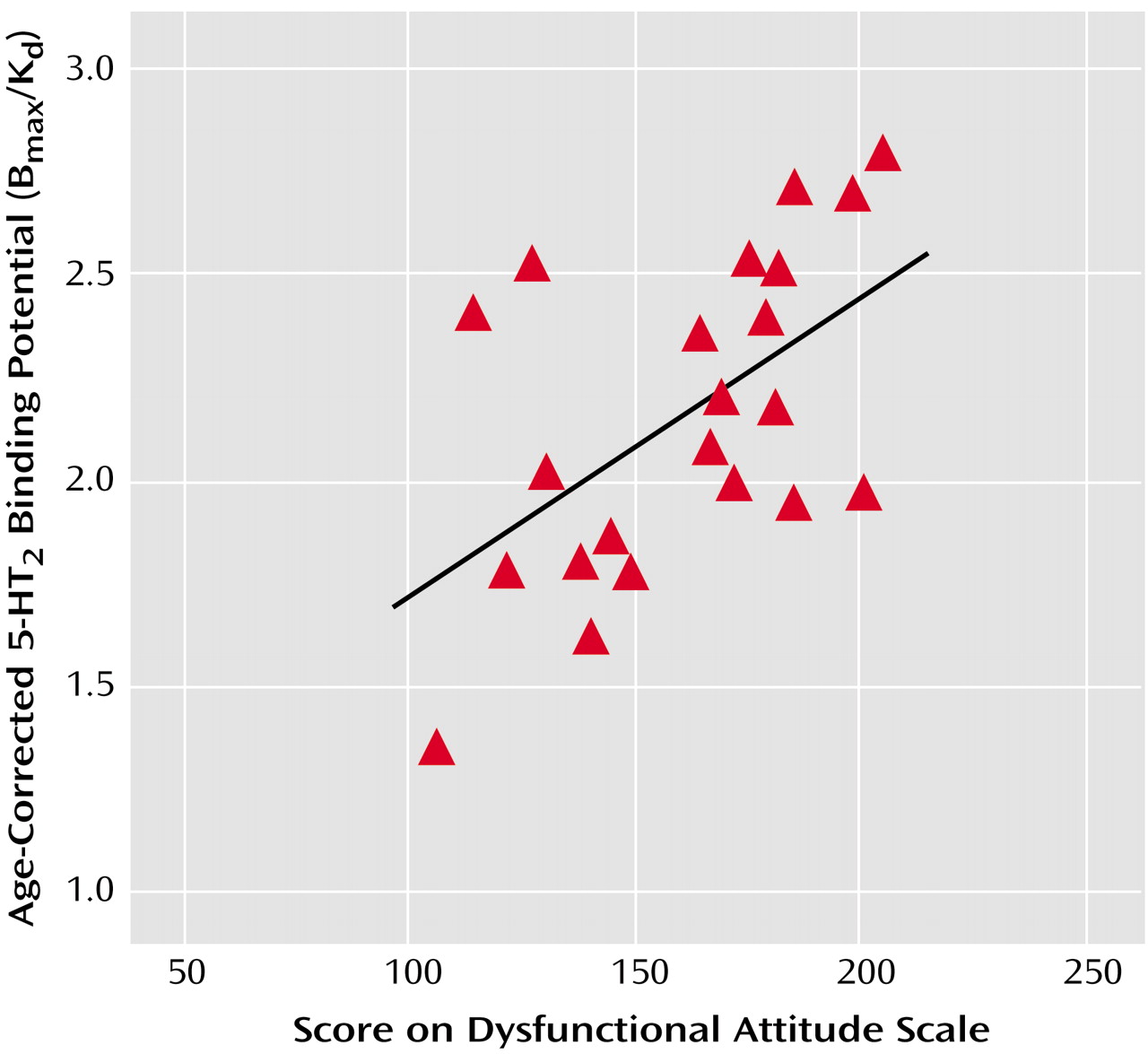
In experiment 2, Dysfunctional Attitude Scale scores covaried strongly with 5-HT
2 binding potential in all cortex brain regions in the patients with a major depressive episode (analysis of covariance [ANCOVA] with Dysfunctional Attitude Scale score and age as covariates—Dysfunctional Attitude Scale score effect: F=4.6 to 9.9, df=1, 19, p=0.04 to 0.005) (
Figure 2). After the effects of age were controlled, the attitude scale scores were significantly associated with 5-HT
2 binding potential throughout the entire cortex (
Figure 3). No such associations were present in self-harming patients with chronic suicidal ideation (ANCOVA with Dysfunctional Attitude Scale score and age as covariates—Dysfunctional Attitude Scale score effect: F=0.5 to 1.5, df=1, 13, p=0.48 to 0.24). The mean Dysfunctional Attitude Scale scores in the major depressive episodes group and the self-injurious group were 162 (SD=32) and 164 (SD=52), respectively. Consistent with previous reports
(7–
13), 5-HT
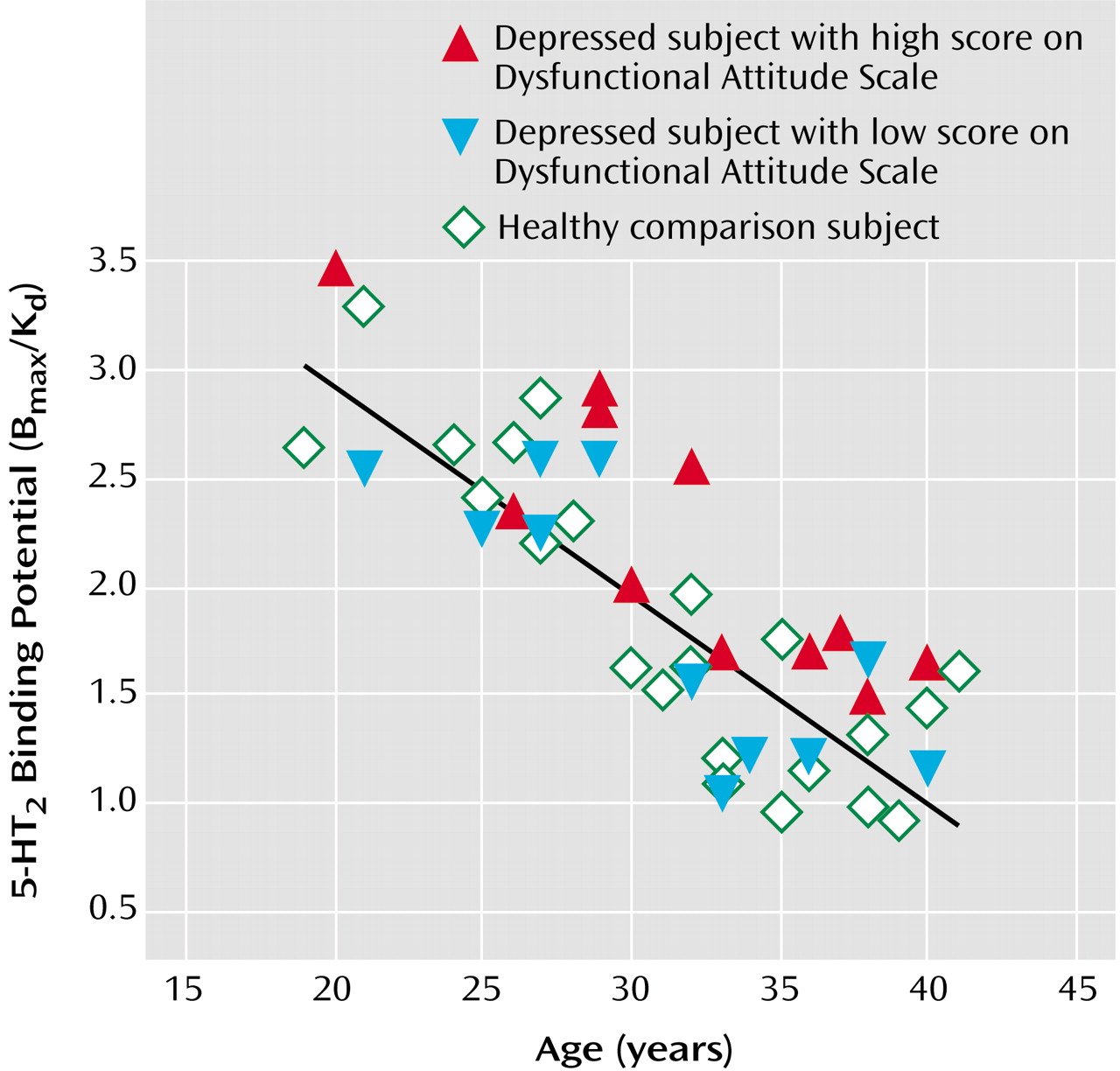
2 binding potential declined with age in the healthy, major depressive episodes, and self-harming groups, as shown in both regional analyses and voxel-based analyses (ANCOVA for regional analyses: F=31.4 to 52.7, df=1, 20, and F=22.2 to 50.1, df=1, 11, p≤0.002 for all analyses; voxel-based analyses: N=80265 to 85333 suprathreshold voxels, p [cluster size]<0.001).
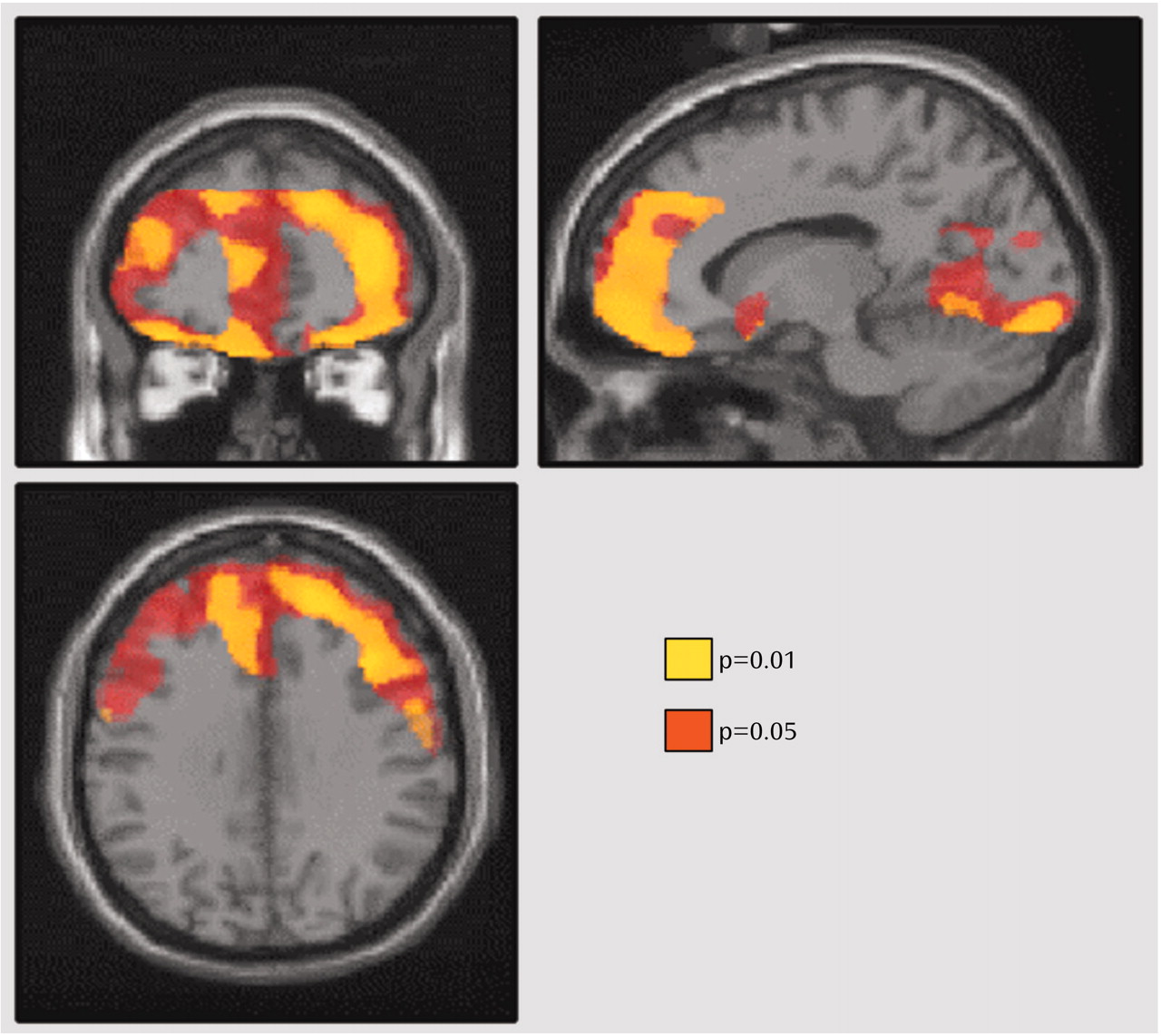
The patients with major depressive episodes were divided into two groups on the basis of whether their Dysfunctional Attitude Scale score was above or below the median score of 166 for the entire group of depressed patients. The subgroup with high Dysfunctional Attitude Scale scores had significantly greater 5-HT
2 binding potential in all brain regions than the age-matched healthy subjects (ANCOVA with group and age as covariates—effect of group [depressed with high Dysfunctional Attitude Scale score]: F=5.1 to 11.5, df=1, 19, p=0.04 to 0.003; effect of age: F=34.4 to 53.7, df=1, 19, p<0.001). In the regions analyzed, the mean 5-HT
2 binding potential was 21% to 29% higher in the high Dysfunctional Attitude Scale score group than in the healthy subjects, with the greatest difference in the middle frontal gyrus (Brodmann’s area 9) bilaterally (29% higher) (
Figure 4).
Within the major depressive episodes group, regional and voxel analyses showed no associations of 5-HT
2 binding potential with score on the suicidal ideation subscale of the Hamilton Depression Rating Scale, overall severity of depressive symptoms (total Hamilton depression scale score), number of previous episodes, duration of depression, past antidepressant use, or any of the five orthogonal factors
(44) within the Hamilton depression scale (mood depression, sleep disturbance, weight loss, somatization, agitation/anxiety).
In regional and voxel-by-voxel comparisons of 5-HT2 binding potential, no significant differences were found between the patients with serious self-harm behavior and the age-matched healthy patients, although 5-HT2 binding potential was a mean of 4% lower in the self-harm group (ANCOVA with age covariate—effect of self-harm: F<0.1 to 1.3, df=1, 33, p=0.98 to 0.27). Two subgroup analyses were done. Patients with a history of more severe self-harm behavior (i.e., stabbing self in chest or more severe behaviors [N=10]) were compared to age-matched healthy subjects, and no significant differences in 5-HT2 binding potential were found, although 5-HT2 binding potential was a mean of 10% lower in the severe self-harm group (ANCOVA with age covariate—effect of severe self-harm: F<0.1 to 2.8, df=1, 17, p=0.79 to 0.11). Patients with a lifetime history of more than five self-harm attempts (N=12) were compared to age-matched healthy subjects, and no significant differences in 5-HT2 binding potential were found, although 5-HT2 binding potential was a mean of 5% lower in the frequent self-harm group (ANCOVA with age covariate—effect of frequent self-harm: F<0.1 to 1.1, df=1, 21, p=0.99 to 0.29).
Discussion
This study had three main findings. The first was that dysfunctional attitudes decreased after administration of d-fenfluramine in healthy subjects. The second was that higher levels of dysfunctional (more pessimistic) attitudes during major depressive episodes were associated with higher 5-HT2 binding potential in the cortex. The third main finding was that patients with major depressive episodes and high levels of dysfunctional (pessimistic) attitudes had higher 5-HT2 binding potential in the cortex, compared to healthy subjects.
The first finding indicates that increasing 5-HT agonism can lower dysfunctional attitudes.
d-Fenfluramine causes an increase in extracellular 5-HT concentration by inducing the neuronal release of 5-HT
(19,
20). The optimistic shift in dysfunctional attitudes after
d-fenfluramine administration demonstrates a role for 5-HT-releasing neurons as modulators of dysfunctional attitudes in humans.
This role of 5-HT-releasing neurons as modulators of dysfunctional attitudes may explain the second finding of an association between dysfunctional attitudes and cortex 5-HT
2 binding potential during major depressive episodes. The converse of the first finding is that lower levels of 5-HT agonism may be related to higher levels of dysfunctional attitudes. Low 5-HT agonism has been shown to up-regulate 5-HT
2 receptors
(5,
6). Thus, low 5-HT agonism during major depressive episodes can account for both an increase in dysfunctional attitudes (toward pessimism) and an increase in 5-HT
2 binding potential: the lower the 5-HT agonism, the greater the increase in both 5-HT
2 binding potential and dysfunctional attitudes. This would create an association between 5-HT
2 binding potential and dysfunctional attitudes, as observed.
This interpretation of low 5-HT agonism resulting in both higher levels of dysfunctional attitudes and higher 5-HT2 binding potential also explains the third finding. Patients with a major depressive episode and greater severity of dysfunctional attitudes have very low 5-HT agonism, and 5-HT2 binding potential in this subgroup is distinguishably higher than that in healthy subjects.
The third finding reflects a key difference in the approach taken in the current study, compared to previous imaging investigations of major depressive episodes and 5-HT
2 binding potential. Previous studies tested the hypothesis that all patients with major depressive episodes have an abnormal 5-HT
2 binding potential
(45–
48). The main drawback of this method is that the diagnosis of major depressive episodes is based on a symptom cluster and individual symptoms of major depressive episodes are not always present. The current study tested the hypothesis that patients with more severe symptoms, as indicated by higher scores on the Dysfunctional Attitude Scale, would show an abnormally high 5-HT
2 binding potential in the prefrontal cortex. Another important aspect of the current study design is that all of the patients were drug-free for at least 1 month and five half-lives of any previous medication. We are aware of only one large study that reported lower 5-HT
2 binding potential during major depressive episodes, and this study selected patients who had recently been treated with antidepressants that increase 5-HT concentrations
(48). To our knowledge, the number of patients with major depressive episodes in the current study is larger than in any previous study in this area. In addition, this study examined two different groups of patients—those with major depressive episodes and those with self-harm behaviors—with respect to a common symptom of dysfunctional attitudes.
The present study suggests a new interpretation for the results of postmortem investigations of suicide victims that have reported higher 5-HT
2 receptor density in Brodmann’s area 9
(7–
13). These findings may represent patients with major depressive disorder and major depressive episodes who have high levels of dysfunctional attitudes. In the present study, the subgroup of patients with major depressive episodes and high levels of dysfunctional attitudes had a higher 5-HT
2 binding potential in the cortex (including Brodmann’s area 9), compared to healthy subjects. The presence of major depressive disorder is the most likely explanation of the postmortem findings, as suggested by previous findings that more than 50% of suicide victims have major depressive episodes secondary to major depressive disorder
(49,
50). In addition, two studies of drug-free suicide victims with major depressive episodes secondary to major depressive disorder found higher 5-HT
2 receptor density in Brodmann’s area 9
(11,
12). Higher levels of dysfunctional attitudes during major depressive episodes may be linked to suicide, as suggested by findings that scores on the Beck Hopelessness Scale are predictive of eventual suicide
(51,
52). Also, in studies with large numbers of patients with major depressive episodes, Beck Hopelessness Scale scores were consistently correlated with Dysfunctional Attitude Scale scores
(3,
53–55). To our knowledge, no study has explicitly addressed whether dysfunctional attitudes are predictive of eventual death by suicide during major depressive episodes.
This new interpretation is incompatible with the reports of two postmortem studies that did not find higher 5-HT
2 receptor density in the temporal cortex of suicide victims
(9,
13). However, this discrepancy could reflect differences in the sensitivity of imaging and postmortem techniques in detecting change in some cortex regions.
Cortex 5-HT
2 binding potential appears to be unrelated to the higher levels of dysfunctional attitudes observed in the patients with chronic self-harm behavior. It is not surprising that the etiology of higher levels of dysfunctional attitudes in self-harming patients with borderline personality disorder would be different from that in patients with major depressive episodes secondary to major depressive disorder. For example, psychological factors can influence dysfunctional attitudes, and the psychological intervention of cognitive behavior therapy has been shown to reduce dysfunctional attitudes in patients with major depressive episodes
(2,
3). If psychological factors are extreme, they could introduce sufficient variance to obscure a relationship between dysfunctional attitudes and serotonin measures. Self-harming patients with borderline personality disorder often report extremely abnormal experiences associated with a long history of relationships with very negative outcomes, including early childhood abuse and/or a lifetime of disturbing short-term relationships
(56,
57).
The lack of a relationship between dysfunctional attitudes and 5-HT
2 binding potential in patients with recurrent self-harm behavior should not be interpreted as ruling out other 5-HT abnormalities that do not influence 5-HT
2 binding potential (i.e., 5-HT lesions
[58] or 5-HT abnormalities in locations separated from 5-HT
2-containing pyramidal cell neurons). It has been reported that a-[
11C]methyltryptophan uptake in cortex is reduced in patients with borderline personality disorder
(59).
In this study, age was a covariate of the cortex measure of 5-HT
2 binding potential. 5-HT
2 receptors are mostly contained in dendrites of pyramidal cell neurons, and the density of pyramidal cell neuron dendrites declines sharply with age over the second to the fourth decades
(60,
61). By covarying the effects of age, we were able to distinguish this and other age-related effects from the effect of illness on cortex 5-HT
2 binding potential.
In this paper we presented a brief overview of the relationship between 5-HT and the 5-HT
2 receptor. However, the available information regarding the regulation of the 5-HT
2 receptor is complicated. In reviewing this information, it is useful to differentiate two categories of relationship: 1) the relationship between 5-HT concentration and 5-HT
2 receptor regulation and 2) the relationship between 5-HT
2-binding medications and 5-HT
2 receptor regulation. In this study, the first of these relationships is most relevant. Earlier studies have demonstrated that increased 5-HT after administration of selective and nonselective monoamine oxidase-A inhibitors is associated with a down-regulation of 5-HT
2 receptors
(17,
18). It has also been demonstrated that decreased 5-HT after administration of reserpine
(5) or the tryptophan hydroxylase inhibitor
p-chlorophenylalanine
(6) is associated with increased 5-HT
2 receptor density. These specific interventions appear to influence the presynaptic storage of 5-HT and consequently increase (monoamine oxidase inhibitor
[62]) or decrease (
p-chlorophenylalanine
[63], reserpine
[64]) extracellular 5-HT. The intact functioning of the synapse may be important for the relationship between 5-HT and 5-HT
2 receptors because lesioning of the 5-HT-releasing neurons does not result in up-regulation of 5-HT
2 receptors
(58,
65). In summary, the relationship between 5-HT concentration and 5-HT
2 receptor regulation is best demonstrated by manipulations of 5-HT that influence presynaptic 5-HT storage in functional neurons (and indirectly influence synaptic concentrations of 5-HT).
As for the relationship between 5-HT
2-binding medications and 5-HT
2 receptor regulation, antagonists are traditionally associated with up-regulation and agonists with down-regulation of postsynaptic, G-protein-coupled receptors
(66). Several agonists for 5-HT
2 receptors are associated with the traditional, expected, down-regulation of 5-HT
2 receptors
(66,
67). At least one antagonist (SR 46349B) is consistently associated with the traditional, expected, up-regulation of 5-HT
2 receptors
(68–
70). However, some 5-HT
2-binding medications with antagonist effects can also down-regulate 5-HT
2 receptors
(66). There is no reason to assume that medications cannot induce conformational changes in the receptor such that the traditional category of agonist and antagonist no longer applies. Some categories of medications have a mix of antagonist, agonist, and other interactions with receptors (partial agonists, inverse agonist, etc.). It has been proposed that 5-HT
2 antagonists with down-regulation properties exert these properties by promoting internalization (traditionally associated with agonists)
(66). The effects of medications on receptor regulation may provide information about how a receptor regulates. However, there is no reason to assume that the interaction between specific medications and a receptor is fully representative of the interaction between the endogenous neurotransmitter and the receptor.
This study had limitations typical of ligand PET and brain imaging studies in humans. 5-HT concentrations in the brain cannot be measured directly in humans; therefore, we used indirect measures and made interpretations about these measures. The binding potential reflects B
max/K
d (density × affinity). Although we are unable to discern between these two parameters, it is likely that an increase in 5-HT
2 binding potential reflects an increase in 5-HT
2 B
max. Postmortem studies of suicide victims report higher levels of 5-HT
2 B
max (7–
13), and animal models of 5-HT depletion report higher levels of 5-HT
2 B
max (5,
6). Even if decreased 5-HT
2 receptor stimulation resulted in an increase in affinity (decrease in K
d), binding potential would still be increased in the same direction, and this result would not confound our main interpretations.
We found higher 5-HT
2 binding potential throughout the cortex. Our interpretation is that the higher 5-HT
2 binding potential can be attributed to a lower level of 5-HT in the cortex with normally functioning 5-HT
2 receptors. This interpretation need not apply to other 5-HT receptor abnormalities reported during depressive episodes. Lower levels of 5-HT
1A binding potential have been found in most cortex regions during depression
(71,
72). Decreased 5-HT transporter density within the prefrontal cortex was found in a large postmortem study of depressed subjects
(73); however, this finding is not consistently reported
(12,
73–76).
The conclusion that low 5-HT agonism is responsible for the association between higher levels of 5-HT2 binding potential and dysfunctional attitudes is the simplest explanation of the highly significant findings of the separate experiments of this study. Usually the simplest explanation (with the fewest assumptions) for multiple observations is the correct one; however, it is possible that more complicated explanations may account for the association between dysfunctional attitudes and 5-HT2 binding potential during depressive episodes. To resolve this issue, dysfunctional attitudes should be measured in future investigations of serotonin abnormalities during depressive episodes.
In summary, this study had several novel findings. Dysfunctional attitudes decreased after administration of d-fenfluramine, suggesting that neuronal release of 5-HT may modulate dysfunctional attitudes in healthy humans. Abnormal functioning of 5-HT modulation during major depressive episodes can explain the association between cortex 5-HT2 binding potential and dysfunctional attitudes: low 5-HT agonism may lead to higher levels both of 5-HT2 binding potential in the cortex and of dysfunctional attitudes. A subtype of major depressive episodes with higher levels of dysfunctional attitudes was identified, and subjects with this subtype had higher 5-HT2 binding potential in the cortex, compared to healthy subjects. These findings indicate an important role for abnormal serotonergic neuromodulation in the pathophysiology of dysfunctional attitudes during major depressive episodes. These findings have significant implications for future research on suicide in depression.