Schizophrenia causes pervasive mental changes that include the ability to think clearly, perceive correctly, and experience and express emotions intensely. The neural basis of its cognitive disturbances (e.g., memory deficits, attentional impairments, hallucinations) has been intensively explored, using the tools of functional neuroimaging
(1–
6). Emotional abnormalities are among the most striking features of this illness. Patients sometimes appear to have lost the ability to feel. For example, they may respond impassively when told of the death of a loved one. They may also have difficulty interpreting the emotional significance of events or situations, which may in turn lead to delusional interpretations of their meaning. The neural mechanisms of emotional disturbance in schizophrenia have been less well investigated than other aspects of the illness
(7,
8). However, several functional neuroimaging studies have begun to examine this issue
(9–
13). In general, but not always
(11), patients with schizophrenia fail to activate limbic and paralimbic regions when either correctly or incorrectly performing affective tasks.
The interpretation of these previous studies is, however, limited because all studies have included patients who were taking different or multiple neuroleptic medications during the functional imaging experiment
(9–
14). The effects of neuroleptic (also known as antipsychotic) medications on affective functioning in schizophrenia are multifaceted. Neuroleptics may either reduce or exacerbate the emotional difficulties of patients, depending on the specific receptor profile and its interaction with affect-regulating neurotransmitter systems. They also have effects on psychopathology, produce neurological side effects, and influence cerebral blood flow (CBF). Therefore, because of the importance of emotion in schizophrenia and to circumvent the confounding of treatment with neuroleptic medications, we conducted a functional imaging study of patients with schizophrenia after a period of withdrawal of neuroleptics of at least 3 weeks. The present study of medication-free patients examined one facet of emotion: the ability to attribute the correct emotional meaning to images (pictures of objects, people, and situations) that are commonly seen in everyday life.
Many studies have now shown that “normal” emotion is not a unitary construct
(15). Processing emotion-laden stimuli has evaluative, experiential, and attributive components. Emotions also have direction and valence, such as positive or negative, pleasant or unpleasant, happy or sad. Phylogenetically, emotions may also have levels, ranging from more primitive emotions that promote survival (fear of dangerous stimuli, sexual desire) to emotions that are “higher” and less obviously adaptive (feeling altruistic, generous, or loving). Animal, human lesion, and functional imaging studies have begun to dissect the various neural substrates of these components of emotion
(16). For example, the subcortical limbic system (including the amygdala) is devoted to the evaluation of negative stimuli, particularly for potential danger
(17). The prefrontal cortex is a pivotal node for the evaluation of pleasant and rewarding stimuli
(18).
We have previously used positron emission tomography (PET) to study the neural correlates of attributing the emotional valence of positive and negative visual stimuli in normal healthy volunteers
(19). We now have extended this work to the study of schizophrenia. Appraisal of the emotional significance of a stimulus is a critical stage in emotional processing, and this ability is often impaired in patients with schizophrenia. In this study, we compared patients’ response to visual images that reflect situations or objects common in everyday life that represent extremes of emotional valence. Regional CBF (rCBF) was measured while 18 patients with DSM-IV schizophrenia and 17 healthy volunteers formulated a judgment (attribution) of the pleasantness or unpleasantness of these stimuli.
Results
Although the patients with schizophrenia had “normal” ratings on the unpleasant pictures, they displayed difficulty in attributing the correct valence to the pleasant pictures. Pleasant stimuli were rated by the patients as mean=3.62 (SD=4.04) and by the comparison subjects as mean=6.11 (SD=1.11) (t=2.44, df=31, p=0.02, two-tailed). On the other hand, the unpleasant stimuli were rated by the patients as mean=–4.37 (SD=4.48) and by the comparison subjects as mean=–4.87 (SD=4.09) (t=–0.32, df=31, p>0.70).
In the patients, direct comparison
(33) of unpleasant and pleasant tasks yielded a paucity of findings, reflecting their muted emotional responsiveness. There was an area of relatively increased CBF associated with response to unpleasant stimuli in the right ventral lateral prefrontal cortex (Brodmann’s area 47, t
max=4.54, 153 voxels; Talairach coordinates: x=32, y=37, z=–10) and one area of increased CBF in the right ventral medial prefrontal cortex (Brodmann’s area 10, t
max=–4.09, 95 voxels; Talairach coordinates: x=2, y=48, z=–18) in response to the pleasant task. Direct task comparisons among healthy volunteers can be found elsewhere
(19).
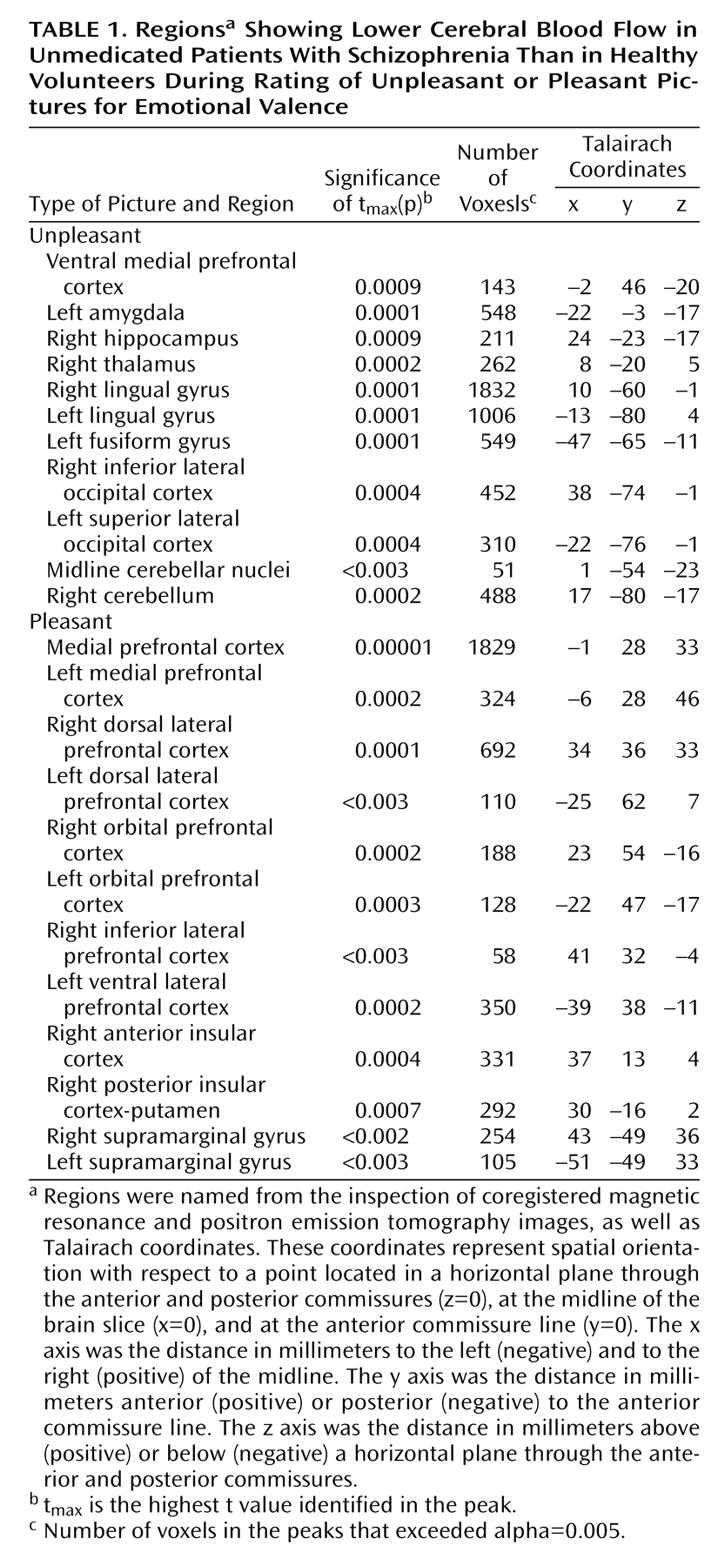
Our randomization analysis indicated that when they were compared with normal healthy comparison subjects, the patients with schizophrenia exhibited widespread decreases in CBF when they assessed and rated the emotional valence of the unpleasant pictures (
Table 1 and
Figure 1). Most of these regions had increased CBF in the normal comparison subjects when they rated the unpleasant stimuli. The normal subjects had activation in the left amygdala, the cerebellum, and the secondary visual cortex in the unpleasant/pleasant comparison
(19). The decreases in CBF in the patients were the inverse of the normal response and reflected a failure to activate the regions normally used in performance of this emotional task.
One group of regions showing an abnormally decreased response to the unpleasant pictures included the limbic and paralimbic structures implicated in the assessment and appraisal of danger: the amygdala, hippocampus, and medial ventral prefrontal cortex. Other regions showing decreased activity in schizophrenia included the visual association cortices. In addition to these decreases in the limbic, paralimbic, and association cortex, the patients also showed decreases in CBF in the thalamus and cerebellum while appraising the unpleasant pictures, indicating that the abnormalities occurring during emotional appraisal arise from a widely distributed network that includes subcortical regions.
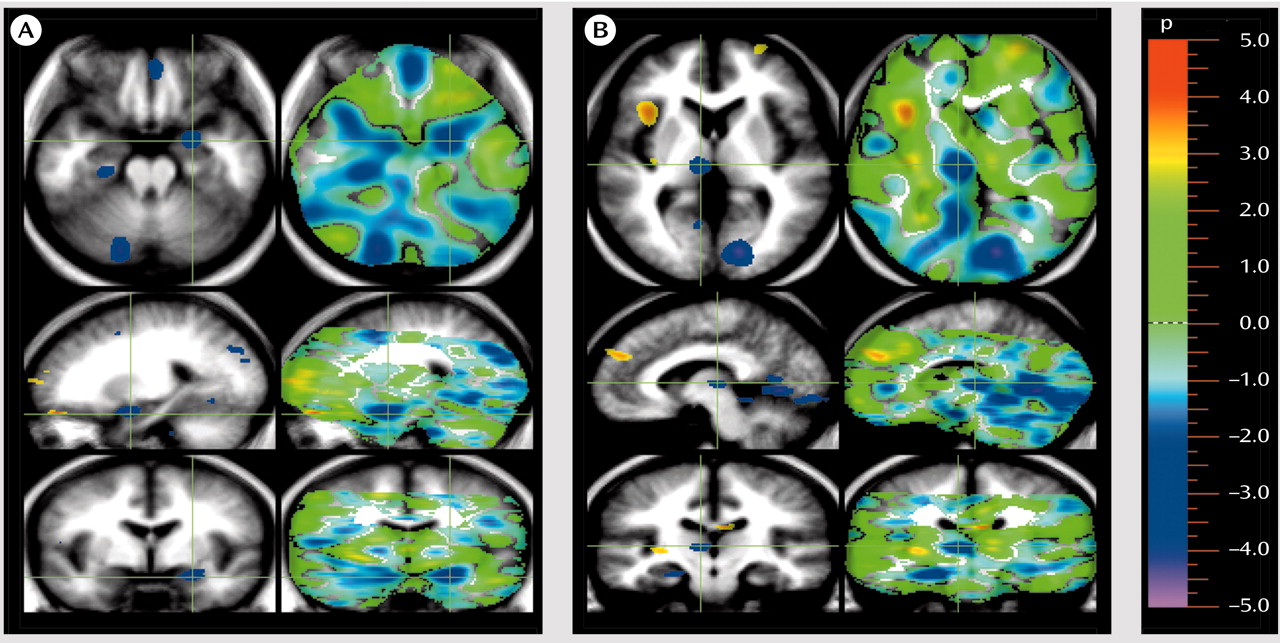
A different gestalt emerged when the CBF patterns of the patients and comparison subjects were compared during appraisal of the pleasant stimuli (
Table 1 and
Figure 2). Again, the pattern observed in the patients was an inverse of the pattern observed in the healthy volunteers. Whereas the healthy comparison subjects showed activations in the medial, orbital, and dorsal lateral frontal cortex
(19), the patients had decreases in multiple regions of the prefrontal cortex, reflecting a lack of activation of the networks used for pleasure recognition and attribution. In addition, they also had decreases in CBF in the insular cortex, an allocortical region that is connected to the limbic system, consists of multimodal sensory integration regions, and is thought to play a role in perceiving relationships between the external world and the internal milieu
(34).
We did not find areas of increased CBF in patients relative to comparison subjects during attribution of valence to either unpleasant or pleasant stimuli.
Correlations were calculated for the relationship between symptom severity and ratings of emotional valence in the patients with schizophrenia. There was a significant inverse correlation between the ratings of the unpleasant pictures and the positive symptom dimension, which is comprised of delusions and hallucinations (r=–0.72, N=16, p<0.002). That is, the more severe the positive symptoms, the higher the tendency to rate the unpleasant stimuli as unpleasant. There was also a significant direct correlation between ratings of the unpleasant pictures and the negative symptom dimension (r=0.55, N=16, p<0.03). That is, the more severe the negative symptoms, the lower the ability to recognize the negative emotional valence of the pictures and instead to interpret them more positively.
Discussion
To our knowledge, this is the first study to report on the functional neuroanatomy of affective functioning of nonmedicated patients with schizophrenia. These extensive decreases in CBF during valence attribution to affectively laden visual stimuli suggest impairment in the systems of the brain used for recognition of the emotional significance of people, situations, and objects. This study confirms the results of previous studies
(9–
14) and extends the findings to nonmedicated patients. This neural response is consistent with the clinical presentation of schizophrenia. The emotional deficits of schizophrenia are well-recognized phenomena
(7,
35–41). However, the neural basis of these deficits has not yet been fully mapped. A pivotal aspect of appropriate affective reactions is the ability to attribute the correct emotional value to events and situations. This study advances the neurobiological understanding of this core aspect of the psychopathology of patients with schizophrenia.
CBF and Neural Response to Unpleasant Stimuli
The patients with schizophrenia were unable to engage neural nodes known to be responsible for processing negatively charged affective stimuli, including the amygdala, the hippocampus, and the ventral medial frontal cortex. Functional imaging studies have reported amygdala abnormalities in patients with schizophrenia who responded with the appropriate affect to sadness-inducing human faces
(42) and in patients who failed to respond to fearful faces
(43). Hence, our study is consistent with previous reports of functional abnormality in the amygdala of patients during probes by negative stimuli. In the present study, the findings concerning the amygdala were left lateralized. This phenomenon may be due to the nature of our stimuli. The human amygdala may respond with right-lateralized activity to affect-laden human faces and with left-lateralized activity to nonhuman scenes
(44) or in circumstances requiring a cognitive representation of fear
(45). Our study helps clarify that the processing of faces is likely not the only causative factor for abnormal amygdala function
(46). Adding to the mapping of amygdala dysfunction is another difference between studies. In the study by Schneider et al.
(42), subjects were asked to rate their subjective mood rather than attribute a valence to stimuli, unlike the study by Phillips et al.
(43) and ours. In agreement with previous studies
(10), in our study, failure to engage the amygdala was consistent with the appropriate attribution of valence. It is worth noting, however, that at least two studies did not show reduced amygdala response to affective stimuli
(9,
11). These findings may be explained on the basis of active neuroleptic medication in patients during task performance.
Animal and human studies have demonstrated that the amygdala plays a key role in the affective evaluation of negative or dangerous situations
(17,
19,
47–53). It has been called “the hub in the wheel of fear,” since it acts as a router that permits a rapid and adaptive response to dangerous stimuli and a slower, more reflective response after the meaning of the stimulus has been assessed
(17). The slow response is mediated by the hippocampus, which responds to unexpected stimuli as part of a novelty-assessment network by retrieving stored contextual and emotional experiences that permit attribution of meaning or valence to the stimuli
(54,
55). The ventral frontal cortex is also part of this emotion appraisal network
(18,
56,
57). Other regions showing decreased activity in schizophrenia include the visual association cortices, which also participate in determination of meaning through recognizing the specific nature of perceptions, such as in faces or objects
(48).
Abnormalities in the thalamus are consistent with a growing number of postmortem, in vivo morphometric, and functional imaging studies that describe primary abnormalities in the thalamus in schizophrenia
(58–
64). Functional imaging studies have also shown that the thalamus is associated with visually induced negative affect
(65,
66).
An abnormality in midline cerebellar activity in patients during an emotional valence recognition task is consistent with the posited role of the cerebellum in emotion
(67,
68). Cerebellar midline structures (i.e., the vermis), together with other older cerebellar regions (i.e., the fastigial nuclei and the floccular nodular lobule) have been regarded as the equivalent of the limbic cerebellum
(69) and have been found to be abnormal in schizophrenia
(70). A large decrease in CBF was also observed, however, in the cerebellar cortex. Failure to recruit the cerebellum, along with other nodes in the cortical-cerebellar-thalamic-cortical circuit, completes an impairment posited to be the central unifying mechanism of symptom production and cognitive disturbance in schizophrenia
(71). Therefore, the present study extends to emotion processing the findings of previous imaging studies obtained by using cognitive paradigms that did not include emotion
(72).
This study did not find areas of relative increase in CBF in patients with schizophrenia relative to comparison subjects in either task. This paucity of activation (the “silent brain”) is consistent with our within-group analysis showing little differential activation in patients responding to unpleasant versus pleasant stimuli. However, consistent with the data of Taylor et al.
(9), small areas of increased CBF were found in the ventral prefrontal cortex in association with correct interpretation of unpleasant stimuli and in response to pleasant stimuli in the within-group analyses of the patients.
CBF and Neural Response to Pleasant Stimuli
Although the normal comparison subjects activated an archaic danger-recognition system when rating the unpleasant pictures, they activated multiple sites in the phylogenetically younger network of pleasure-recognition sites in the prefrontal cortex and in the insular cortex when rating the pleasant pictures
(19). In contrast, as shown in
Table 1 and
Figure 2, the patients with schizophrenia had pervasive decreases in CBF in multiple regions of the prefrontal cortex while attempting to attribute the correct emotional valence to pleasant stimuli. These results are consistent with numerous studies that have previously demonstrated both structural and functional abnormalities in the prefrontal cortex in schizophrenia
(73–
84).
The patients also had decreased CBF in the insular cortex while attempting to attribute the correct emotional valence to pleasant stimuli. Decreased CBF in the insula in patients with schizophrenia is consistent with previous studies that have shown functional and structural abnormalities in the insular cortex in schizophrenia
(85–
87). The insular cortex is a multimodal integration region that is involved in the linking of sensory experiences with their appropriate emotional responses
(88,
89). Previous functional neuroimaging studies have reported abnormalities in CBF in the insula during diverse emotional states, including the experiencing of aversive stimuli, nociception, anticipatory anxiety, and mood provocation
(89–
92). Insular gray matter volume and cortical surface size are inversely related to the severity of psychotic symptoms in schizophrenia
(93).
These results indicate that patients with schizophrenia have at least two facets of neurofunctional deficits. The first one, which might be called condition independent, has been observed in patients, regardless of the mental task at hand, and entails deficits in frontal-thalamic-cerebellar circuitry. The results of this study are similar to those obtained in others in which patients performed “pure” cognitive tasks
(94,
95). On the other hand, condition-specific deficits were also observed during this study of emotion. These were seen in regions governing affect regulation. Condition- or task-specific neurofunctional deficits are often found together with task-independent deficits in functional neuroimaging studies of schizophrenia
(26,
96).
Clinical Significance
The results observed at the neural level, by means of measurements of CBF, are consistent with the clinical picture of schizophrenia, especially when examined in the context of the ratings of emotional valence given by the patients. The blunting of subjective experience and emotional expression are common symptoms in schizophrenia and are clinically referred to as part of the group of negative symptoms (anhedonia, asociality, and affective blunting). The patients in this study had difficulty in attributing the correct valence to pleasant pictures. They lacked the “normal” ability to recognize pleasant stimuli. However, they were able to attribute correctly an unpleasant or negative valence to the unpleasant pictures. Maintaining an intact behavioral response to aversive stimuli conveys survival advantages for people with schizophrenia, but their inability to recognize and attribute a positive valence for pleasant pictures is reflective of the suffering that they experience during survival.
Examination of the correlations between symptom patterns and the ability to attribute the correct emotional valence to the stimuli added to the clinical perspectives of this study. The patients who had higher levels of positive symptoms (delusions and hallucinations) were more likely to give the unpleasant pictures a more negative rating, seeing them as extremely unpleasant. This suggests that these patients had an unusual sensitivity to negative stimuli, making them more vulnerable to misinterpretation of their emotional meaning. This unusual sensitivity may form part of the basis for misperceptions and delusional interpretations. On the other hand, the patients who had higher scores on the negative dimension (i.e., anhedonia, affective blunting, asociality) also had difficulty in giving the correct (negative) rating to the unpleasant pictures, instead giving them a relatively more positive rating. This correlation between severity of negative symptoms and an inability to recognize the negative emotional valence of unpleasant pictures may explain other facets of the clinical experience of schizophrenia. Specifically, this rating pattern is consistent with clinical manifestations, such as both the blunting of affect and inappropriate affect.
In summary, we found that when patients evaluated the unpleasant pictures, they did not engage the limbic and paralimbic regions used by the healthy volunteers, even though they correctly rated them as unpleasant. The patients also failed to activate the areas of the prefrontal cortex that are normally used to recognize images as pleasant, but in addition, they were unable to recognize them as pleasant. This network of decreases in CBF during attribution of a positive or negative valence to pleasant or unpleasant visual stimuli in patients with schizophrenia suggests a failure in the functional brain systems used for recognition of the emotional significance of people, situations, and objects.