Persons suffering from schizophrenia show multiple deficits, and it has been a goal of schizophrenia research to develop unifying, core mechanisms that can parsimoniously explain such diversity.
There is solid neuropsychological and imaging evidence that temporofrontal function is disordered in schizophrenia
(1–
5), suggesting that core deficits may be localized in that region. One candidate core function is working memory
(6), a capacity-limited system that enables the holding of information while the individual works on a problem
(7). Information is held transiently after the sensory input is ended, and the representations are used to plan or decide on a course of action
(8). In its basic form, the model includes a “central executive” (or supervisory attentional system)
(9), which allocates between sensory representations depending on the informational analysis required for the specific task, and two “slave” systems, a phonological loop and a visuospatial scratch pad
(7), which retain specialized information over brief periods of time. In the model, executive functions are located in the prefrontal cortex and storage is more posterior, in the motor and sensory systems
(10).
Because of the central role of working memory, malfunction of this system can be logically perceived to cause impaired goal-oriented behavior, disorganized cognitive organization, failure of self-monitoring, and other phenotypic manifestations of schizophrenia.
There is abundant evidence that schizophrenia patients perform poorly on tests of working memory
(5,
8,
11–15), and several studies showed correlations between working memory deficits and other neurocognitive abnormalities, including processing speed
(16,
17) and executive function
(18,
19). However, to our knowledge it has not yet been demonstrated that working memory dysfunction underlies diverse neuropsychological functions served by different neural systems in the same individual, as predicted for a core dysfunction. Furthermore, since phonological and visuospatial aspects of working memory are distinct, differential relations of these with other neuropsychological functions should be observed.
To provide these data and test the hypothesis that working memory is a core dysfunction in schizophrenia, we studied the relationship between working memory and a range of cognitive, motor, and emotional functions.
The term “working memory” is used in different ways
(20), which may contribute to inconsistencies in findings
(15,
19). We chose to study a basic component of working memory, “on-line storage,” by using the digit span tests from the Wechsler Adult Intelligence Scale
(21) to measure the capacity of the phonological loop and the Dot Test
(22) to measure the visuospatial scratch pad. Performance on these tests is impaired in schizophrenia
(22,
23), and their simplicity makes them less vulnerable to interference from nonspecific factors such as lack of motivation or uncooperativeness. To reduce intersubject variability, we included only stable, chronically ill male patients treated with atypical antipsychotics.
The pattern of relationships between working memory and other functions was compared with that found in well-functioning normal healthy subjects.
Method
Subjects
The participants were 27 men suffering from chronic schizophrenia, which was diagnosed by consensus between treating and research clinicians who were all senior psychiatrists and who used clinical interviews, observations, and case notes to determine the DSM-IV diagnosis. All of the patients were treated with atypical antipsychotics: olanzapine (N=9, mean dose=16.39 mg/day, SD=4.86), risperidone (N=15, mean dose=4.97 mg/day, SD=1.74), or clozapine (N=3, mean dose=225.00 mg/day, SD=28.87). Each patient had received the same medication for at least 3 months, and the dose was unchanged for at least 4 weeks before the study.
The patients’ mean age was 38.0 years (SD=13.5), and their mean education level was 11.2 years (SD=3.0). They had been ill for a mean of 12.7 years (SD=10.5), and their current hospitalizations had lasted a mean of 4.5 years (SD=9.5). The patients in the study group were considerably impaired and had had limited response to treatment with typical and atypical antipsychotics. Many were candidates for discharge to hostels and other supported community accommodation, which was becoming increasingly available at the time. Patients with scores lower than 20 on the Mini-Mental State Examination (MMSE)
(24) were excluded. Testing was done over 2–3 sessions totaling 3–4 hours.
A high-functioning comparison group (16 men and 22 women) consisted of healthy professional staff at the center. Their mean age was 42.4 years (SD=11.1); this was not significantly different from the mean age of the patient group, but the comparison subjects were significantly better educated; their mean education level was 15.2 years (SD=1.6) (t=5.70, df=63, p<0.001). As the performance of the male and female participants did not differ significantly, they were pooled for analyses.
All participants signed written informed consent statements after receiving a full explanation of the test procedures. The study was approved by the institutional ethics committee.
Clinical Assessments
The clinical rater was independent of the neuropsychological tester and unaware of test results.
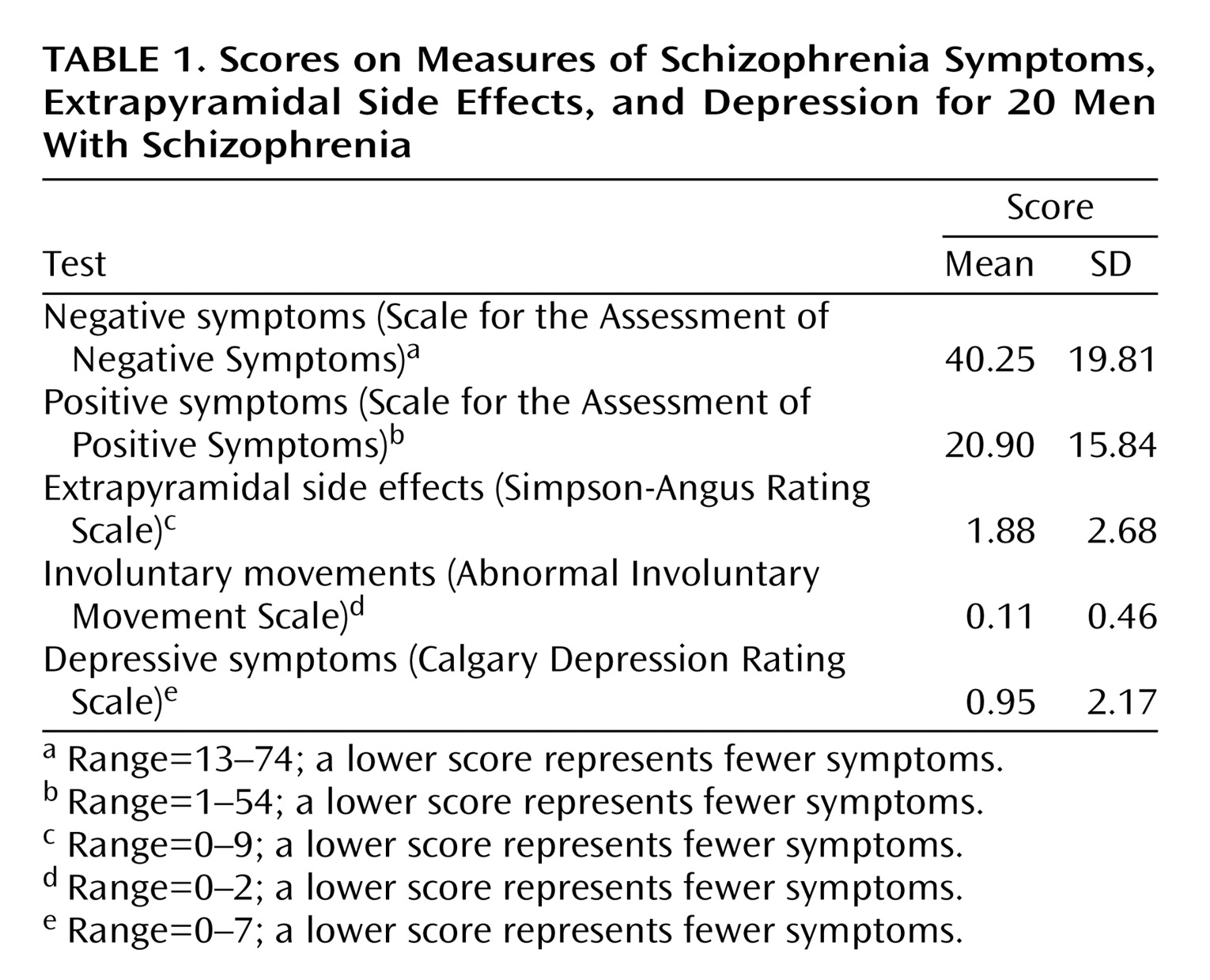
Negative and positive symptoms were assessed by using the Scale for the Assessment of Negative Symptoms (SANS)
(25) and the Scale for the Assessment of Positive Symptoms (SAPS)
(26), and extrapyramidal side effects were measured with the Simpson-Angus Rating Scale
(27) and the Abnormal Involuntary Movement Scale
(28). Depressive symptoms were assessed with the Calgary Depression Rating Scale
(29), which was developed for use with schizophrenia patients. The scores for 20 of the patients are shown in
Table 1.
Neuropsychological Tests
Digit span
The forward and backward digit span tests from the WAIS, version 1
(21), were used.
Dot Test
We used a modification of the Dot Test
(22). In the original test the subject is presented with a card on which a dot is present; to facilitate measurement, we used a cross instead of a dot. After a 10-second interval the paper is removed and the subject is asked immediately to reproduce the mark on a blank card. The distance between the target mark and that recalled by the subject is measured in millimeters. This procedure was repeated for a total of 10 cards. The sum for all 10 cards was the outcome measure.
Benton Visual Retention Test
The Benton Visual Retention Test
(30) tests the subject’s ability to reproduce visually presented designs. Ten cards with designs are presented one at a time for 10 seconds and then removed. The subject is asked to immediately and exactly reproduce the designs. The number of correct responses was the outcome measure.
MMSE
General cognitive function was tested with the MMSE
(24).
Finger Tapping Test
The Finger Tapping Test
(31,
32) examines the ability to make rapid repetitive movements. The test was modified, and the patients were asked to tap with the index finger on two points set 30 cm apart as rapidly as possible. Each hand was tested separately. The outcome measure was the number of taps per minute with the dominant hand.
Visuomotor coordination
Visuomotor testing
(33), examining the ability to coordinate visual and motor functions in the absence of direct eye-hand contact, was performed by using a computerized system consisting of a digitizing tablet and a desktop computer. A general index calculated by combining tracking and tracing errors in the dominant hand was used as the performance measure.
University of Pennsylvania battery
Selected tests from the University of Pennsylvania neuropsychological battery
(34) were used. These computerized tests were designed to yield quantitative measures of performance on behavioral domains that can be linked to regional brain function and were developed on Macintosh computers using the Power Laboratory platform
(34,
35). To limit the number of outcome measures, a representative measure from each test was chosen for analysis. The tests used were as follows.
Abstraction, inhibition, and working memory task
This test
(18) was designed as a measure of abstraction and concept formation with and without additional working memory loads. It presents subjects with five shapes: two in the upper right and two in the upper left corner of a computer screen, with a fifth target object appearing in the center of the screen, below the other stimuli.
The participant’s task is to pair the target object with the objects on either the left or right. In half the trials, an additional requirement for working memory maintenance is superimposed on this basic module by adding a delay between the presentation of the target and other objects. The total number of correct responses for trials without working memory requirements was selected as the performance measure.
Face memory test
The University of Pennsylvania face memory test
(36) consists of 20 target faces and 40 foils (20 for each test trial). The stimuli are black-and-white photographs of faces balanced for gender and age. All faces are of neutral emotional expression, as determined by 12 raters. The total number of true positive responses for short delay periods was the performance measure.
Visual object learning test
This test
(37) was designed as a spatial analogue of the California Verbal Learning Test
(38). It uses 20 Euclidean shapes as learning stimuli, which are presented over four learning trials, followed by short- and long-delay test recall.
New distracter shapes are used in every test trial. The total number of true positive responses for short-delay tests was selected as the performance measure.
Computerized judgment of line orientation
This task
(39) is a computerized adaptation of the original paper-and-pencil task. Participants are shown two lines at an angle and are asked to indicate the corresponding lines on a simultaneously presented array. The difficulty is defined by the length of the stimulus lines. The number of correct responses was chosen as the performance measure.
Continuous performance test
The University of Pennsylvania continuous performance test is a measure of sustained attention
(40) developed for use in functional neuroimaging studies. During this task the participant is asked to respond to a set of vertical and horizontal lines (a seven-segment display) whenever they form a digit. Since each judgment is made on the basis of the present stimulus, working memory demands are minimized. The total number of true positive responses was the performance measure.
Identification of facial emotions
The University of Pennsylvania emotion acuity test
(41) contains 40 black-and-white pictures depicting happy, sad, and neutral facial emotional expressions. The participant is asked to rate the emotional valence of each face on a 7-point scale representing very happy, moderately happy, somewhat happy, neutral, somewhat sad, moderately sad, and very sad. The mean number of correct responses was the performance measure.
Data Analysis
The data were not distributed normally and could not be normalized by transformation, and so nonparametric methods were used. The linear relationships between tests were assessed by using Spearman correlation coefficients. To allow testing of differences between correlations in the schizophrenia and comparison groups, a newly developed nonparametric method for testing differences between “correlated correlations,” or correlation analysis of variance (CORANOVA), was used
(42). The test scores of the comparison and patient groups were compared by using t tests. Two-tailed significance tests, with the significance level set to 5%, were used.
Not all patients completed all tests, and therefore the group sizes differ slightly. A goal of this and future studies is to unravel underlying causal relationships, such as paths from working memory to positive and negative symptoms. Path analysis is an optimal procedure for this task, but the number of subjects in this study precluded its application to the present study data.
Results
Neuropsychological Functioning in Patients and Healthy Subjects
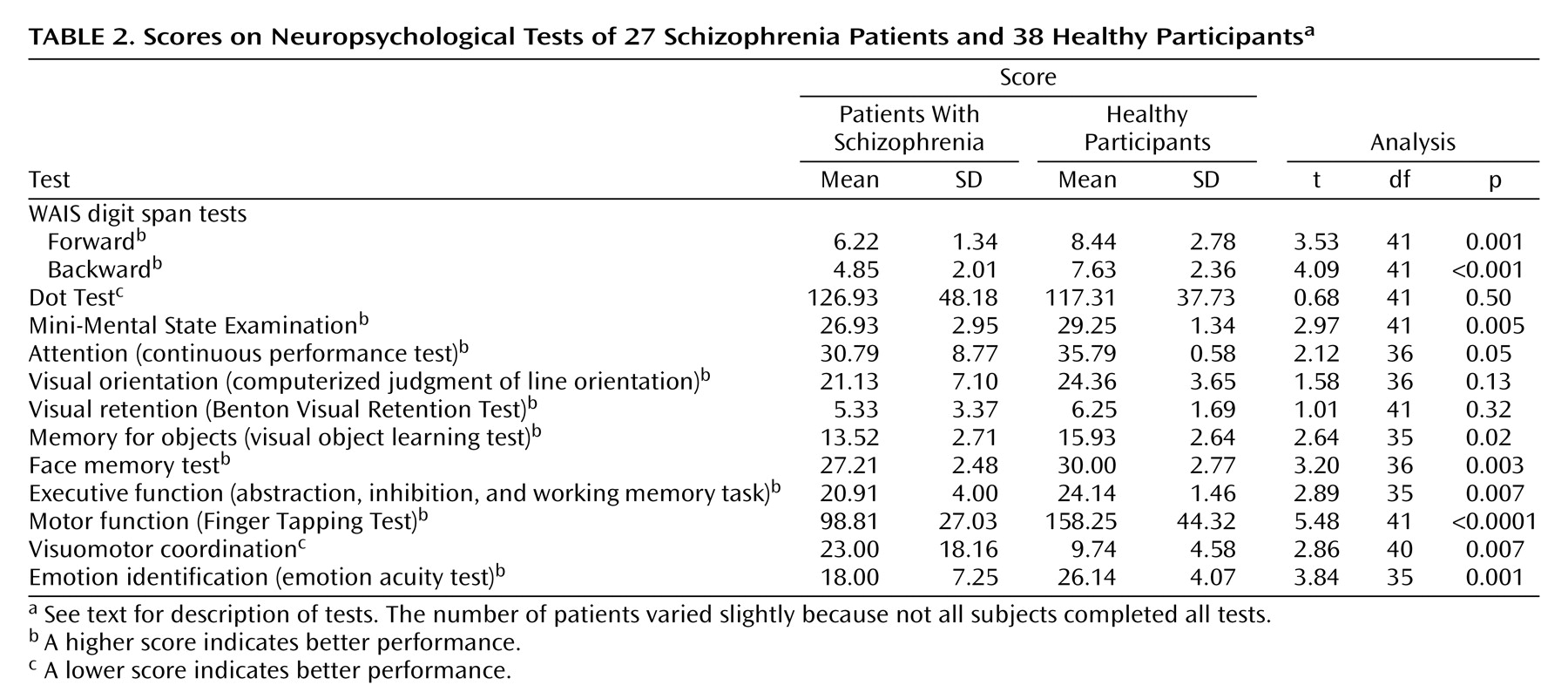
The test scores for the patient and normal groups are shown in
Table 2. The patient group showed significantly poorer performance on all tests except those for spatial working memory (Dot Test), visual orientation, and visual retention.
Correlations of Working Memory With Other Neuropsychological Functions
The scores for working memory of the healthy subjects showed no significant correlation with scores on any of the other neuropsychological tests nor with age or education of the participants.
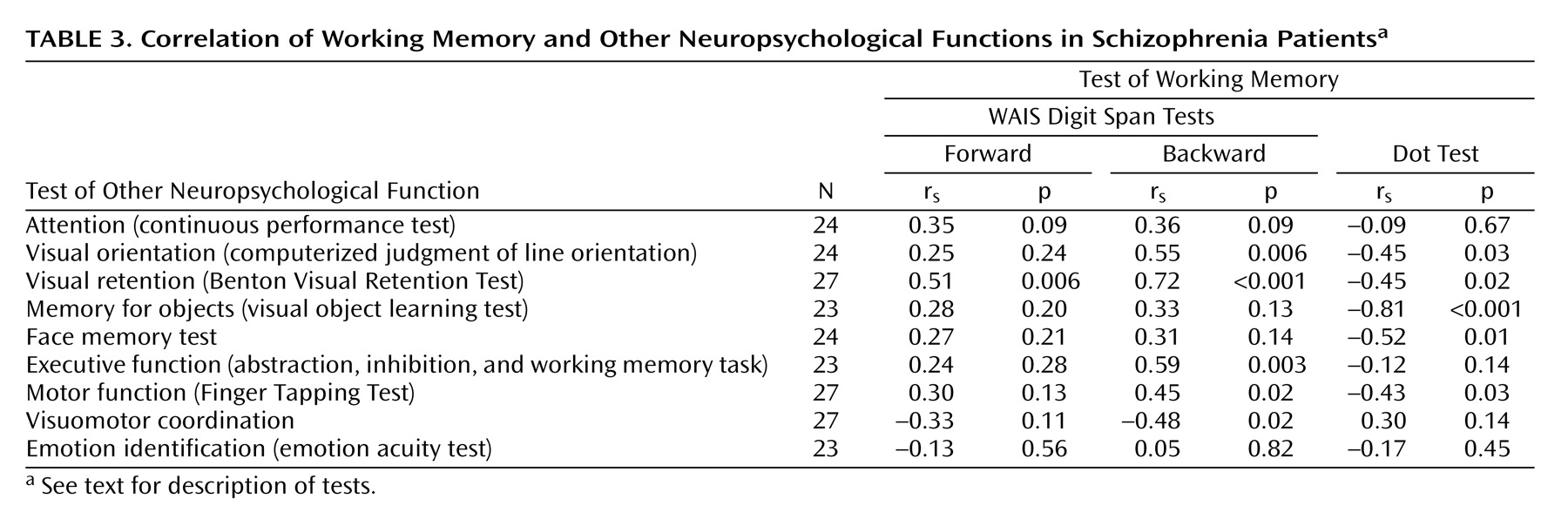
The correlations between working memory and other neuropsychological functions for the patient group are shown in
Table 3. Significant correlations were found between the backward digit span and visual orientation, visual retention, executive function, simple motor function, and visuomotor coordination. The forward digit span showed a significant correlation only with visual retention. Spatial working memory (Dot Test) showed significant correlations with visual orientation, visual retention, memory for objects, memory for faces, and simple motor function but not attention, executive function, or visuomotor coordination. None of the working memory measures correlated with identification of emotions or attention.
Relations Between Different Types of Working Memory
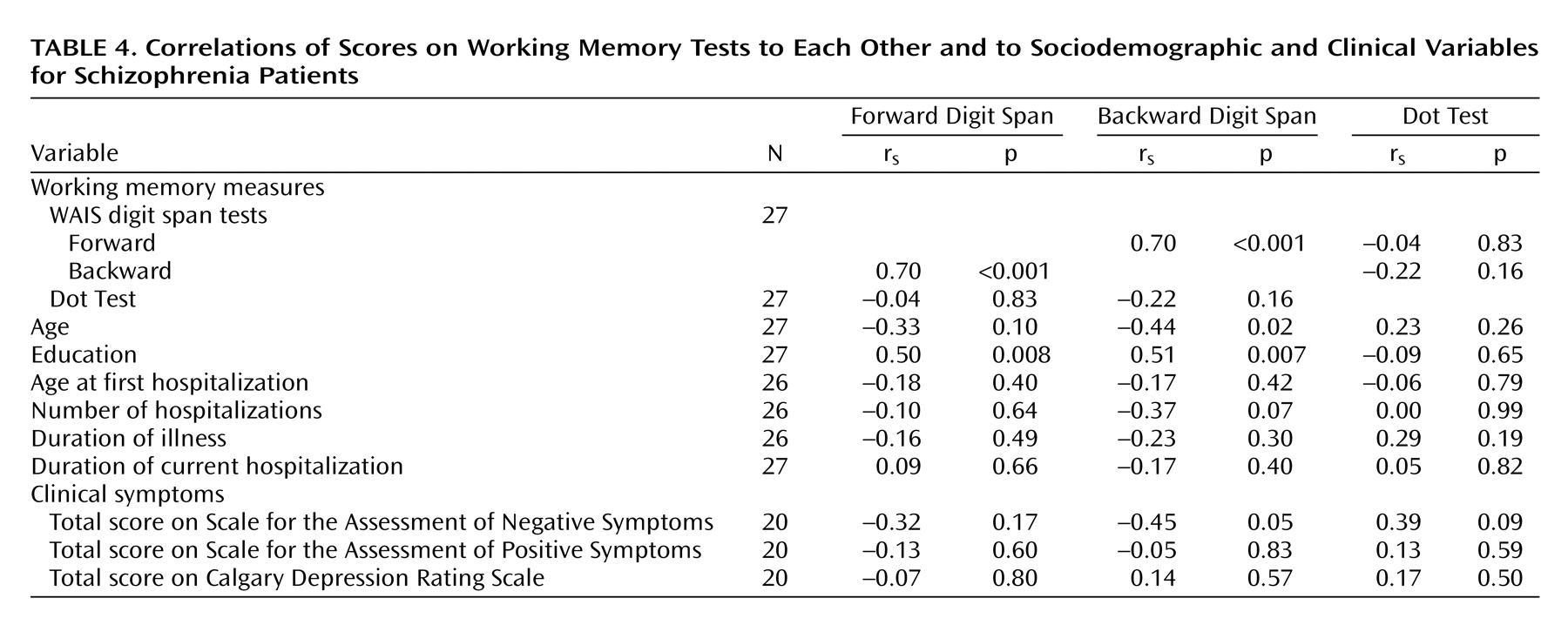
As expected, performance on the backward digit span was poorer than on the forward digit span (
Table 2), and a strong correlation between the two scores was found for the patients with schizophrenia (
Table 4). There was no significant correlation between scores on the forward or backward digit span and on the Dot Test (
Table 4). The forward digit span showed significant correlations with fewer other neuropsychological tests than did the backward digit span (
Table 3).
Correlations of Working Memory With Clinical Data
Scores on the backward digit span showed a significant correlation with negative symptoms (SANS) but not positive symptoms (SAPS) or scores on the Calgary Depression Rating Scale (
Table 4). The scores on the forward digit span and Dot Test did not show a significant correlation with any clinical symptom score. Both the backward and forward digit spans showed significant positive correlations with education level, while only the backward digit span showed a significant, negative correlation with age (
Table 4).
Patterns of Correlations in Patient and Healthy Groups
The individual correlations alone are not sufficient to compare all of the underlying relationships. CORANOVA
(42) was used to compare the patterns of correlations within and between groups as well as to test for differences in the patterns of correlations between groups, or interactions of the correlations. The first set of CORANOVA models addressed unadjusted correlations (with no partialing for age) of the backward digit span with each of the neuropsychological tests, with separate correlations for each group, schizophrenia patients and healthy comparison subjects. Two differences were detected in these correlations, differences between groups in the correlations of the other neuropsychological tests with the Dot Test (p=0.02) and differences in the correlations of the other neuropsychological tests with the Dot Test within each of the groups (p=0.02). That is, the correlations between the other neuropsychological tests and the Dot Test were on average different for the schizophrenia and comparison subjects, and the correlations between the other neuropsychological tests and the Dot Test averaged over the two groups differed across the neuropsychological tests.
It is desirable to be able to assess whether working memory deficits underlie neurocognitive deficits in schizophrenia. One approach to assessing this is through path models, which examine the underlying causal relationships. Unfortunately, a path model is well beyond the scope of analyses that can be considered with 15 observations, the number of male schizophrenia subjects in our data set for whom we have all of the measurements required for a path model. The guidelines on group size for path models are similar to those for regression. Kline
(43) stated that 10 to 20 times as many cases as parameters is optimal for fitting these models and that five times as many or less is insufficient for significance testing of model effects for fitting causal modeling.
Discussion
Our findings support the hypothesis that impaired working memory underlies diverse impairments in schizophrenia. Working memory, verbal or spatial, showed significant correlations with a range of neuropsychological functions, including visual orientation, visual retention, memory for objects, memory for faces, executive function, and simple motor and complex sensorimotor function. Verbal working memory also showed a significant correlation with negative symptoms, consistent with findings in the literature
(15).
Our interpretation of the data is that patients’ lower working memory capacity is “rate limiting” in performance of other cognitive operations. Interaction between capacity and task demand is a performance characteristic of limited-capacity systems. Dreher et al.
(44) found that increasing the set size in a spatial memory task decreased performance in both normal subjects and patients with schizophrenia, with a greater detrimental effect on the patients. Our findings are consistent with those of Gold et al.
(14), showing that auditory working memory capacity is a critical determinant of performance on the Wisconsin Card Sorting Test, and of Glahn et al.
(18), who demonstrated that storage processes are important in the relationship between working memory and executive function. The finding that the patients’ scores on the forward digit span showed a less extensive pattern of correlations than did scores on the backward digit span, which demands more working memory resources, supports our interpretation and is consistent with the findings of Goldberg et al.
(23) that the backward but not forward digit span differentiated between schizophrenia probands and their clinically unaffected identical twins.
Comparison With Normal Subjects
The lack of correlation between working memory and other neuropsychological functions in normal subjects can be explained by postulating that the test demands were well within their working memory capacity (excess capacity) and hence not rate limiting. This was also reflected in the lower score variability in that group, which may have contributed to the lack of correlations between tests.
Since working memory capacity is also limited in normal subjects
(45), it can be predicted that increasing working memory demands to threshold capacity will result in correlation patterns in normal subjects similar to those observed in patients.
Dissociation Between Verbal and Spatial Working Memory
A second major finding was that verbal working memory and spatial working memory scores were dissociated. This is consistent with the hypothesis that verbal and spatial working memory act as two separate scratch pads and with domain specificity of storage systems
(46).
Spatial working memory, but not verbal working memory, correlated with memory for objects and for faces, indicating a close link to systems storing object and face memories. It is noteworthy that spatial working memory performance was highly correlated with memory for objects and faces even though the patients, as a group, did not have impaired performance on the Dot Test. This supports the notion of working memory as a rate-limiting factor in performance. More specifically, given the significance of the difference between this correlation and the correlation of verbal working memory with memory for objects and faces, the results indicate that each type of working memory limits performance on associated cognitive modalities. Evidence from lesion
(47–
49), PET
(50,
51), and functional MRI
(52,
53) studies indicates that perception of faces and of objects is localized in specific systems, including the fusiform gyrus and ventral occipitofrontal cortex, although it is not yet clear to what extent this results from innate organization or is acquired through learning
(54). Transient memory for faces is associated with activity within the inferotemporal cortex, a brain region concerned with recognizing faces
(55,
56). The systems may be lateralized to the right hemisphere
(57).
While only spatial working memory correlated with memory for faces and for objects, both spatial and verbal working memory correlated with visual orientation and retention. This is consistent with a proposed subdivision of spatial working memory into object-based and spatial components, which may be functionally and anatomically separable
(58). There is evidence that spatial working memory functions in nonhuman primates are mediated by regions of the dorsolateral prefrontal cortex that differ from those mediating object working memory functions
(59). This distinction is supported by imaging studies in humans
(60,
61).
Verbal Working Memory and Executive Functions
Verbal working memory showed significant correlations with executive function and visuomotor control, as reported in many studies
(15); this finding is consistent with the location of working memory and executive functions in the prefrontal cortex. Verbal working memory did not correlate with scores on the University of Pennsylvania continuous performance test, indicating that working memory capacity and not attention was the limiting factor. This is consistent with findings that digit span abnormalities in schizophrenia are more likely to be due to capacity limitation than to attention deficiency
(62).
Spatial working memory did not correlate with executive function, indicating that it is not a limiting factor in performance of executive tasks even when the input is visual. This observation is consistent with the finding of Pantelis et al.
(63) that the relationship between visuospatial working memory and errors in performance on an executive function test (“Tower of London”) by schizophrenia patients was due to differences in the strategies used and not spatial memory capacity.
Limitations
A strength of this study is the relatively homogenous nature of the patient group and the extensive range of neuropsychological functions tested simultaneously.
Being a correlation analysis, this study cannot establish a causal relationship between working memory and other functions, and the existence of a common mediating factor cannot be excluded. We considered a series of path models of this relationship with neuropsychological status measurements as mediary variables, but the number of subjects was inadequate. It is unlikely, however, that nonspecific factors such as lack of motivation or cooperation can explain our findings, as the patients’ cooperation was good and their motivation and performance were similar for the simpler and more demanding tests. Likewise, nonspecific influences are not likely to explain the different patterns of correlations for verbal and spatial working memory.
The effect of medication is unlikely to be a significant confound, as all patients received atypical antipsychotics, which minimally affect and may even improve cognitive function in schizophrenia
(64).
The selected nature of the study group, however, limits generalization to other schizophrenia patients.
Conclusions
Our findings support the hypothesis that working memory is a core dysfunction in schizophrenia. They are also consistent with current views that verbal and visuospatial information is held in different systems (domain specificity) and with separation between subsystems for visual objects and visual spatial data. They suggest that in chronic schizophrenia the limited capacity of verbal and spatial “on-line storage” is rate limiting in performance of other cognitive functions. Executive functions rely critically on the phonetic loop, complex visual functions such as object and face recognition depend on the spatial on-line storage system (visuospatial scratch pad), while some complex functions such as visual orientation depend critically on both the verbal and spatial scratch pads.
Further studies are needed to examine whether these relationships are also present in less severely ill patients and to investigate whether the differences between ill and normal subjects reflect differences in performance specifications of similarly organized neural systems or qualitative differences in neural organization and function. Larger study groups would permit application of path analysis to test directional models.