Changes in frontostriatal-limbic function appear to be related to the course of depression
(1). Increased metabolism of the anterior cingulate is a predictor of favorable antidepressant response
(2,
3). Antidepressant treatment decreases the metabolism of the anterior cingulate, and this decrease is associated with favorable response
(4).
To examine further the relationship of the anterior cingulate to change in depressive symptoms during antidepressant treatment, we studied the error negative waves produced during the response inhibition task of the Stroop test; error negative wave amplitude occurs when errors are committed. Frontal error negative waves are thought to reflect cingulate activity
(5). This study tested the hypothesis that frontal error negative wave amplitude during Stroop activation distinguishes depressed elders who remain symptomatic from those who achieve remission.
Method
The subjects were 22 consecutively recruited patients who were older than 60 years and who had nonpsychotic major depression (Structured Clinical Interview for DSM-IV) and a 21-item Hamilton Depression Rating Scale score of 17 or higher. All subjects signed written informed consent. Exclusion criteria were a Mini-Mental State Examination score lower than 24, a history of failure to respond to an earlier antidepressant trial of at least 4-week duration during the current episode, color-blindness, and corrected vision of lower than 20/40 in either eye.
Cognitive impairment was assessed with the Mattis Dementia Rating Scale
(6), which tests five domains, i.e., attention, memory, construction, conceptualization, and initiation/perseveration. Initiation/perseveration includes tasks of category fluency, alternating movements, and graphomotor design. Treatment consisted of citalopram at a target daily dose of 40 mg. Remission was defined as Hamilton depression scale ≤10 at the end of 6 weeks of treatment.
Average evoked potentials were recorded before treatment initiation using NEUROSCAN (Neuroscan, Inc., Herndon, Va.). They were obtained from left (F3, C3, P3) and right (F4, C4, P4) paramedian electrode sites, according to the International 10-20 System, referred to linked-mastoid electrodes, and grounded to the forehead. Electrodes placed at the outer canthi and 3 cm above and below each eye were used to control for eye movement artifacts. The Stroop interference task, used for activation, consisted of equal numbers of the words “RED,” “BLUE,” and “GREEN” (324 words) in a congruent or incongruent color that was presented for 500 milliseconds (at 2-second intervals). The subjects were instructed to identify the color of each word and to press a keypad. A signal was given for each error and when responses were delayed longer than 1.5 seconds. An “error negative wave” was defined as the largest negative deflection within 150 msec after a response.
Comparison of the six error negative waves between remitted and unremitted subjects was conducted with multivariate analysis of variance (MANOVA) to test the overall group effect, lead effect, and lead-by-group interaction effect. Post hoc comparisons between error negative wave means of individual leads were performed with Bonferroni-corrected t tests. Pearson correlation with Bonferroni correction was used to study the association among error negative waves, Hamilton depression scale scores, and Mattis Dementia Rating Scale initiation/perseveration scores. Chi-square and t tests were used to compare clinical and demographic characteristics.
Results
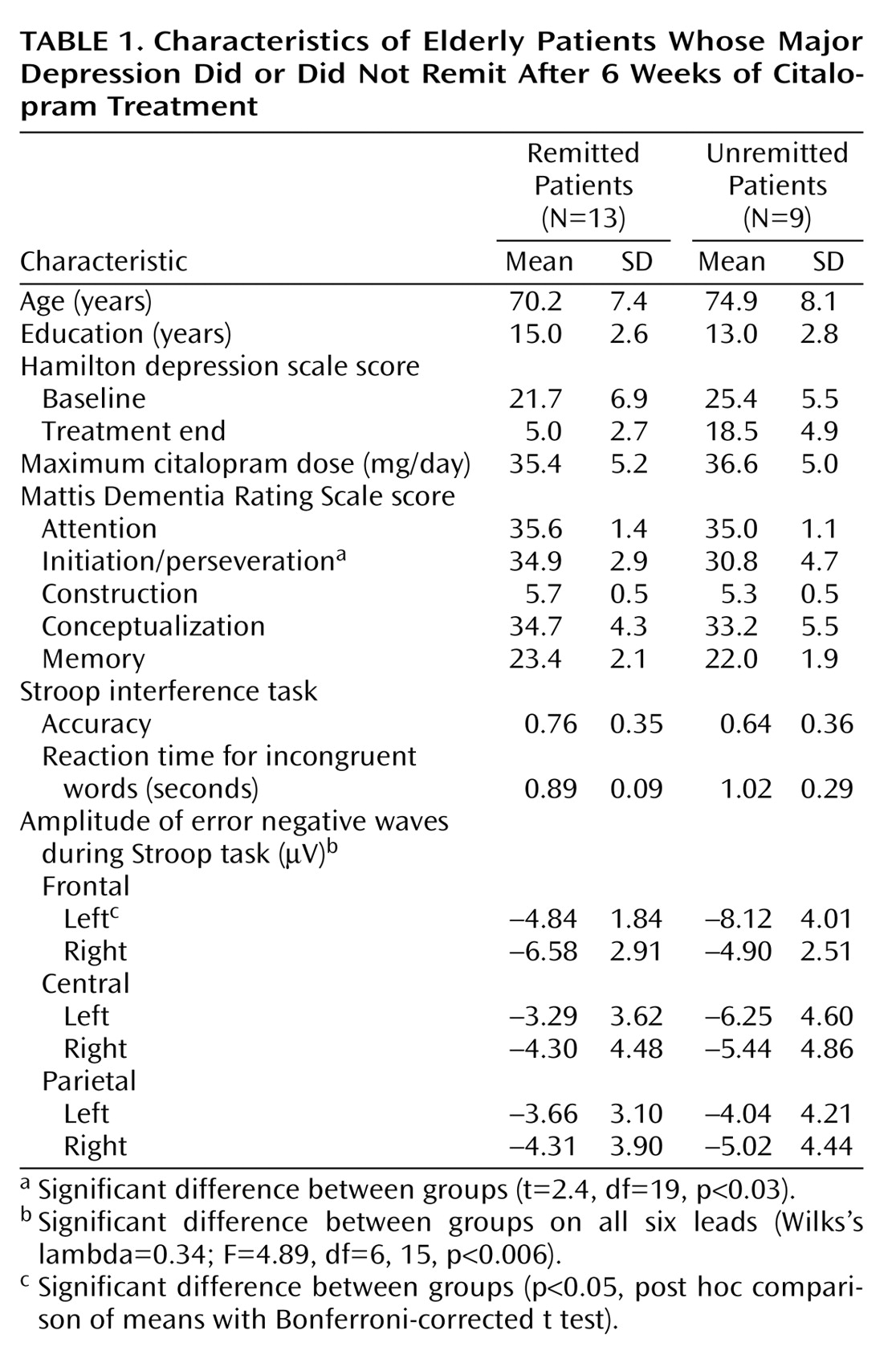
At the end of treatment, 13 patients met criteria for remission and nine remained symptomatic. There were no significant differences in demographic or clinical characteristics between remitted and unremitted patients (
Table 1). Remitted and unremitted patients performed the activation task with comparable accuracy (
Table 1) (t=1.33, df=20, p<0.21) and received treatment of similar intensity (
Table 1) (t=0.92; df=20, p<0.40).
MANOVA showed a significant overall group effect (Wilks’s lambda=0.34; F=4.89, df=6, 15, p<0.006), a significant lead effect (Wilks’s lambda=0.41; F=4.66, df=5, 16, p<0.008), and a significant group-by-lead interaction effect (F=6.24, df=5, 16, p<0.01), indicating that the significance of group effect depends on the location of the lead. The difference in the left frontal error negative wave between the unremitted and remitted patients exceeded that required by Bonferroni-corrected t tests (3.28 versus 2.64) that are required for significance at the p=0.05 level. There were no significant differences in any other paramedian error negative wave. Amplitude of left frontal F3 error negative waves was correlated with percent change of depressive symptoms (according to the Hamilton depression scale) from baseline (r=0.56, df=21, p<0.01). Neither the right frontal F4 error negative wave (r=0.03, df=21, p=0.99) nor any other paramedian error negative wave was associated with percent change in depressive symptoms. Amplitude of left frontal F3 error negative wave was correlated with initiation/perseveration score (r=–0.52, df=20, p<0.02). Initiation/perseveration score was lower (abnormal) in unremitted patients than in remitted patients (t=2.42, df=19, p<0.03). Neither the amplitude of right frontal F4 error negative waves nor that of other paramedian error negative waves was associated with initiation perseveration (r=0.06, df=20, p<0.81).
Discussion
The principal finding of this study is that depressed elders with large left frontal error negative waves after a response inhibition task have limited or slow change in depressive symptoms during treatment with citalopram. Large frontal error negative waves may reflect greater neuronal recruitment during task performance since there were no differences in errors committed during the test. A large number of errors in left frontal error negative waves was correlated with initiation/perseveration impairment, an abnormality also associated with limited or slow change during antidepressant treatment
(1).
To our knowledge, this is the first report of a relationship between error negative wave EEG response to a task mediated by the anterior cingulate and change in depressive symptoms occurring during treatment. This observation is consistent with neuroimaging studies demonstrating an association between abnormal cingulate metabolism and poor antidepressant response
(1–
4).
The association of abnormal initiation/perseveration to limited change of depressive symptoms replicates earlier findings
(1). This observation, along with the correlation of initiation/perseveration scores with left frontal error negative waves, further suggests that anterior cingulate dysfunction contributes to slow or limited change in depressive symptoms as functions tested by the initiation/perseveration require prefrontal pathway integrity including integrity of the anterior cingulate
(7,
8).
The pathophysiology of depression is complex and includes abnormalities not limited to a single region. However, the lateralizing error negative wave effect is consistent with left-side abnormalities of the cingulate and other limbic regions in depression-related states. Depressed mood and psychomotor retardation are associated with low metabolism of the left angular gyrus and the left dorsolateral cortex
(9), structures connected with the cingulate and the amygdala. Threatening words activate the left posterior cingulate gyrus
(10). Dysphoric stimuli activate the left more than the right amygdala, a structure reciprocally connected to the anterior cingulate
(11). Finally, a decrease in the metabolism of the anterior cingulate during treatment predicts a favorable response
(4).
Limitations of this study include the small number of subjects that might have prevented identification of differences in other leads, the lack of a placebo arm, and the short treatment trial. While a longer treatment trial would have been desirable, recent studies suggest that the vast majority of depressed elders who fail to improve significantly by the fourth week of treatment do not achieve remission by 12 weeks
(12). Despite these limitations, the identification of lateralized electrophysiological abnormalities can guide studies of neural systems associated with change in depressive symptoms during treatment.