Working memory deficits are among the constitutive neuropsychological deficits of schizophrenia, and functional imaging studies have consistently indicated dysfunctional activation of the dorsolateral prefrontal cortex during working memory in patients with schizophrenia
(4,
5). Proton magnetic resonance spectroscopic imaging (
1H-MRSI) of
N-acetylaspartate (a marker of neuronal integrity) also has provided evidence of prefrontal neuronal pathology
(6–
9) (in the latter two studies, data were acquired mostly in prefrontal white matter) as well as pathology in other brain regions, although the results have not been without controversy
(10,
11). However,
N-acetylaspartate signals derived from
1H-MRSI are sensitive to treatment with antipsychotics
(12–
15) and also may reflect the functional state of neurons
(16–
21). Indeed,
N-acetylaspartate levels in the dorsolateral prefrontal cortex correlate with physiological activity within the cortical working memory network and have been found to correlate with ratings of negative symptoms in patients with chronic illness
(22,
23). The large majority of
1H-MRSI studies have been performed in chronically ill patients and thus are potentially confounded by issues associated with duration of illness and pharmacological treatment. Studying patients with schizophreniform disorder represents a strategy to control for these factors
(24).
Results
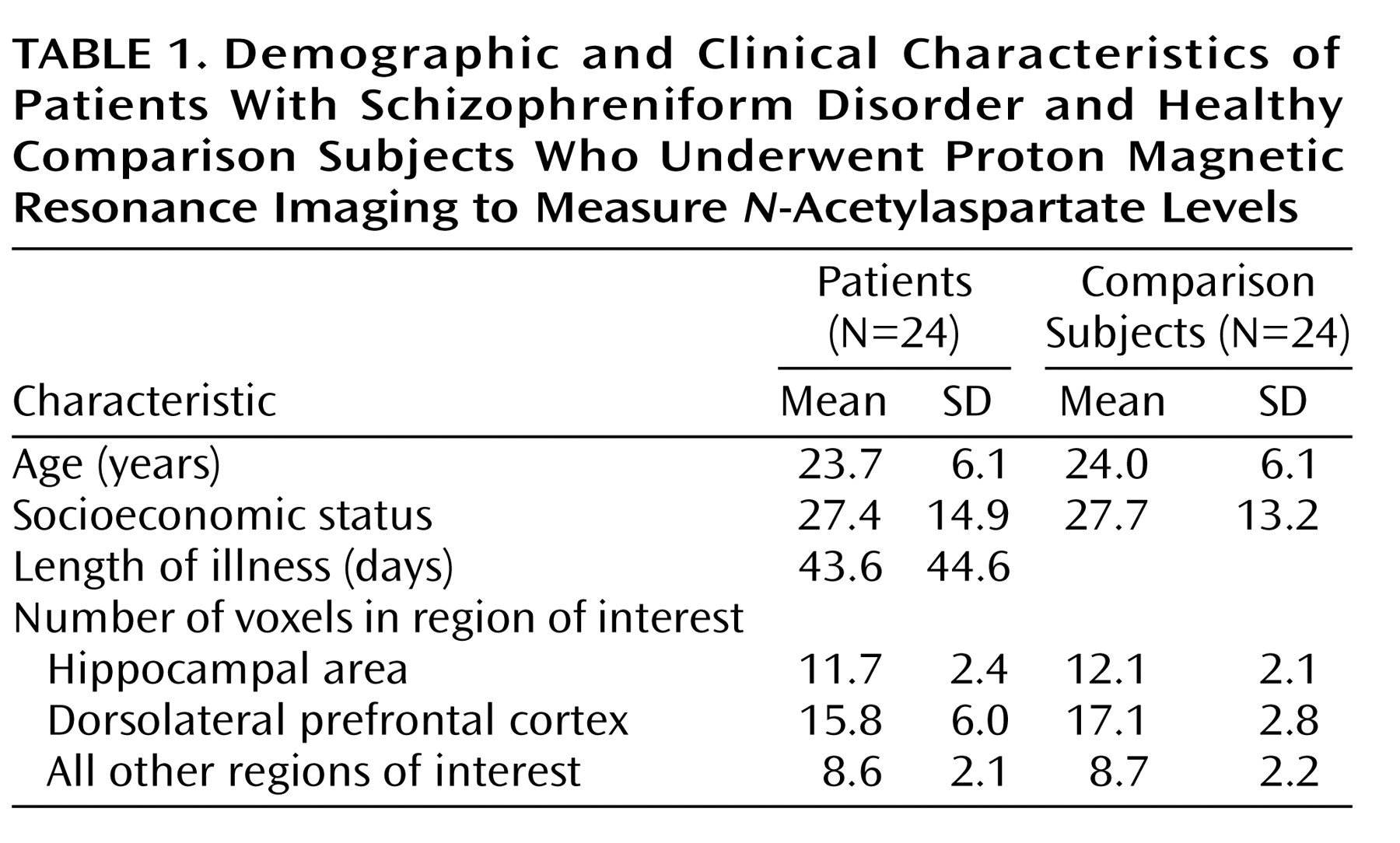
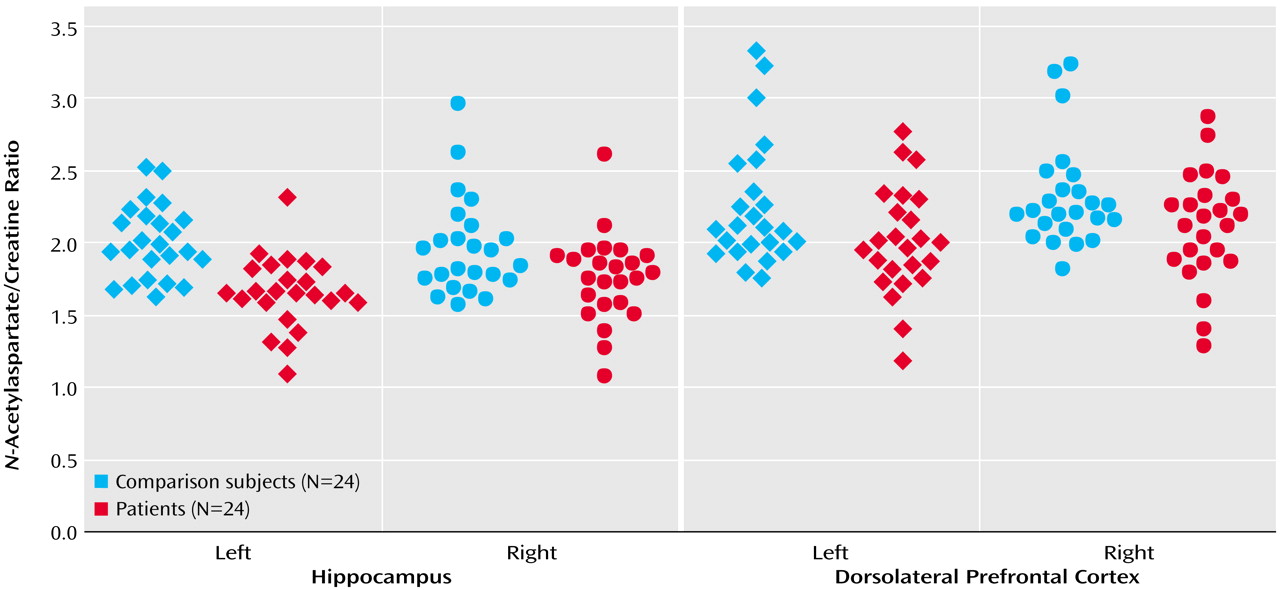
In the hippocampal area, the patients with schizophreniform disorder and the healthy comparison subjects differed significantly in terms of the
N-acetylaspartate/creatine ratio (mean=1.71 [SD=0.34] versus 1.97 [SD=0.29], respectively; F=10.3, df=1, 46, p<0.003) and the
N-acetylaspartate/choline ratio (mean=1.06 [SD=0.24] versus 1.18 [SD=0.19]; F=4.06, df=1, 46, p<0.02). Post hoc analysis showed that patients had a significant bilateral reduction of
N-acetylaspartate/creatine (p<0.03) (
Figure 1) and
N-acetylaspartate/choline (p<0.02). No main effect of hemisphere was found for
N-acetylaspartate/creatine or
N-acetylaspartate/choline. No main effect or interaction was found for choline/creatine.
Likewise, in the dorsolateral prefrontal cortex, the patients with schizophreniform disorder and the healthy comparison subjects differed significantly in terms of the
N-acetylaspartate/creatine ratio (mean=2.05 [SD=0.38] versus 2.32 [SD=0.51], respectively; F=4.44, df=1, 46, p<0.05), and the between-group difference for the
N-acetylaspartate/choline ratio approached significance (mean=1.54 [SD=0.25] versus 1.69 [SD=0.45]; F=2.2, df=1, 46, p=0.10). Post hoc analysis revealed that patients had a significant bilateral reduction of
N-acetylaspartate/creatine (p<0.05) (
Figure 1) and a difference in
N-acetylaspartate/choline that approached significance (p=0.10). No main effect of hemisphere was found for either
N-acetylaspartate/creatine or
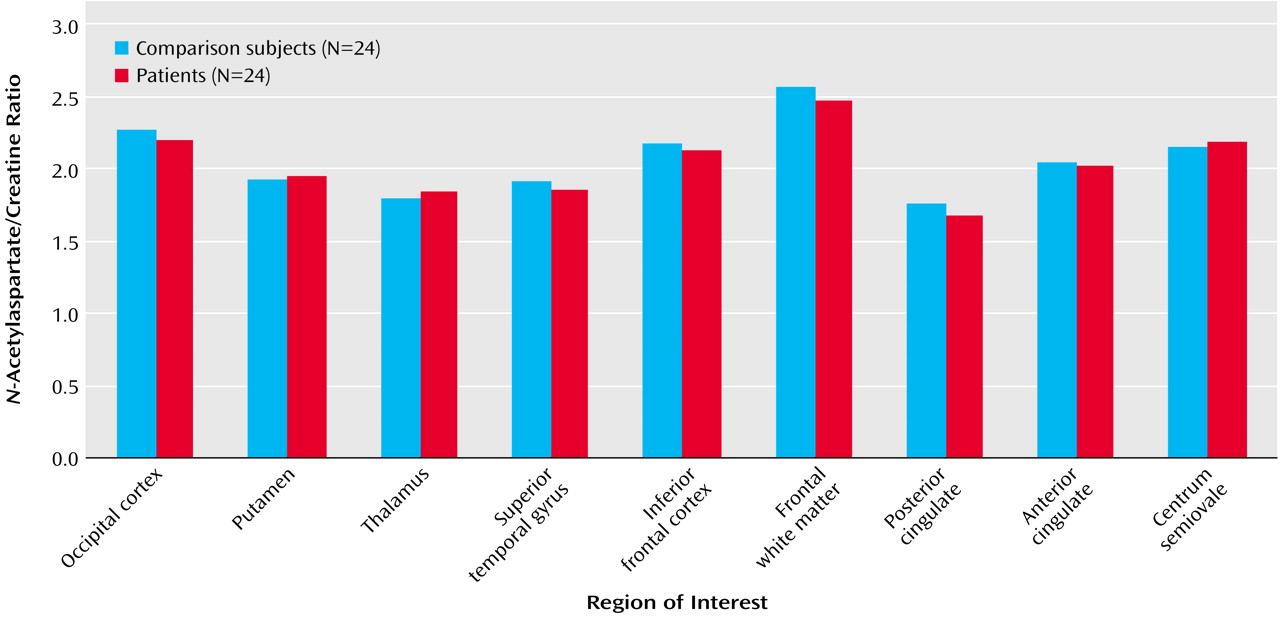
N-acetylaspartate/choline. No main effect or interaction was found for choline/creatine. For all metabolite ratios, no main effect or interaction was found in any other region of interest (results for the
N-acetylaspartate/creatine ratio are shown in
Figure 2). ANOVA revealed sporadic effects of hemisphere and no diagnosis-by-hemisphere interaction. None of the sporadic results, which were not based on a priori hypotheses or previous results, survived an appropriate Bonferroni correction for the number of regions, even at the p=0.10 threshold.
Repeated-measures ANOVA on the N-back data showed a significant effect of diagnosis (F=20.5, df=1, 26, p<0.001), a significant effect of task condition (F=62.7, df=2, 52, p<0.001), and a significant interaction between diagnosis and task condition (F=8.4, df=2, 52, p<0.001). Post hoc analysis showed that patients had lower accuracy than comparison subjects at the 1-back condition (mean=9.26 [SD=3.23] versus 13.7 [SD=0.41], respectively; p<0.01) and the 2-back condition (mean=6.9 [SD=2.4] versus 9.6 [SD=2.8]; p<0.01) but not at the 0-back condition (mean=13.5 [SD=0.9] versus 13.9 [SD=0.1]; p>0.50).
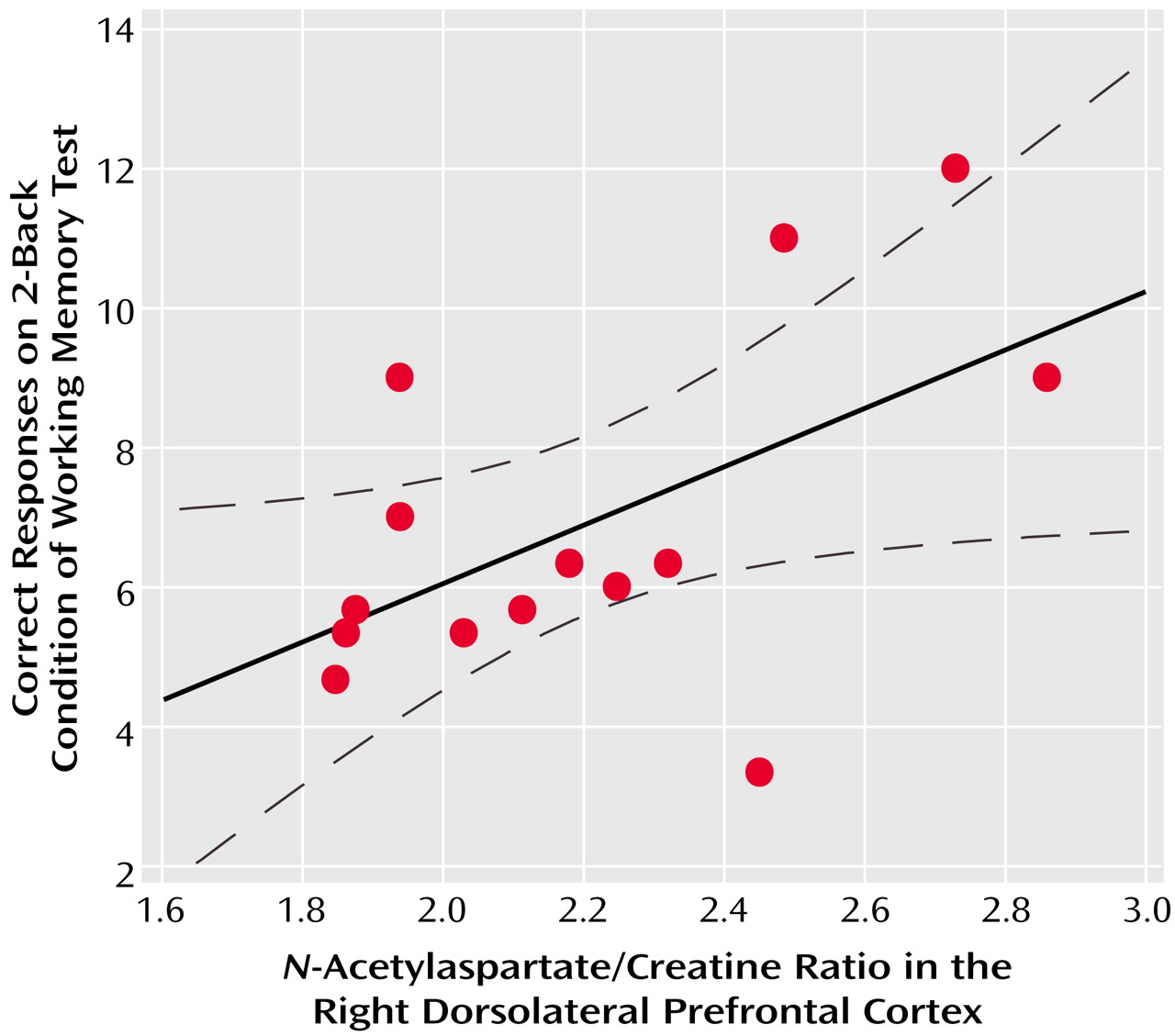
In the patients with schizophreniform disorder, there was a significant correlation between
N-acetylaspartate/creatine in the right dorsolateral prefrontal cortex and working memory performance during the 2-back condition (
Figure 3). A similar relationship was found for
N-acetylaspartate/choline (r=0.39, df=12, p=0.10) but not for choline/creatine (r=0.03, df=12, p=0.90). For the healthy subjects, the correlation between
N-acetylaspartate/creatine in the right dorsolateral prefrontal cortex and working memory performance during the 2-back condition was in the same direction but was not significant (r=0.23, df=12, p=0.30). There were no other significant correlations between any
1H-MRSI measures and N-back performance. Consistent with previous reports (14), we found a correlation between
N-acetylaspartate/creatine in the right dorsolateral prefrontal cortex and negative symptom ratings from the Positive and Negative Syndrome Scale (r=–0.37, df=22, p=0.04, one-tailed). No other correlation was found between
1H-MRSI measures and clinical parameters (age, length of pharmacological treatment, chlorpromazine equivalents).
Discussion
We found reductions of
N-acetylaspartate levels in the hippocampal area and the dorsolateral prefrontal cortex of patients with schizophreniform disorder compared with healthy subjects. We also found a specific correlation between
N-acetylaspartate levels in the right dorsolateral prefrontal cortex and performance on the 2-back version of a working memory task in patients with schizophreniform disorder. This correlation is consistent with findings from an earlier schizophrenia study
(5) in which correlation between
N-acetylaspartate levels in the dorsolateral prefrontal cortex and performance and activation (assessed with functional magnetic resonance imaging [fMRI]) during a 2-back working memory condition was found only on the right. The lack of a statistical correlation between
N-acetylaspartate levels in the dorsolateral prefrontal cortex and performance on the 0- and 1-back conditions as well as between
N-acetylaspartate levels in the hippocampal area and working memory performance is also consistent with earlier studies and suggests that a threshold of sufficient functional load is necessary to elicit a predictable effect
(5).
Our results are consistent with previous studies of chronic patients with schizophrenia that showed
N-acetylaspartate reductions in the hippocampal area and dorsolateral prefrontal cortex. Therefore, neuronal pathology in these two brain areas, at least as reflected in diminished
N-acetylaspartate measures, is not an epiphenomenon of being chronically ill or of being chronically treated with antipsychotics. It is also of interest that
N-acetylaspartate levels in the dorsolateral prefrontal cortex correlated only with performance during the 2-back condition in the schizophreniform disorder patients. The functional neuroimaging literature in schizophrenia has indicated that dorsolateral prefrontal cortex dysfunction becomes consistently manifest if patients are engaged in tasks that depend on prefrontal strategies. The likely complexity and redundancy in prefrontal neuronal processing would be expected to dilute the predictive impact on prefrontal function of
N-acetylaspartate measures alone. Our data are consistent with this contention in that the correlation is only found at the higher level of working memory load. Lack of statistical correlation between
N-acetylaspartate levels in the hippocampal area and working memory performance is also consistent with this idea and with earlier studies
(5).
The correlation between
N-acetylaspartate levels in the dorsolateral prefrontal cortex and performance during the 2-back condition of the working memory test did not reach statistical significance in the comparison subjects, even though it was in the same direction as in patients. Earlier
1H-MRSI work in healthy subjects had shown that
N-acetylaspartate levels in occipitoparietal white matter correlated positively with an index of general neuropsychological performance
(26) and with full-scale IQ measured with the Wechsler Adult Intelligence Scale
(27). Both of these studies involved a larger number of subjects (N=43 and N=26, respectively) and a decidedly different brain region. Our data are partially consistent with this earlier work in that no significant correlations were seen in the healthy subjects, even though the direction was the same. Lack of consistency with this earlier work may be ascribed to several reasons. One possibility is that we studied a smaller number of subjects. Another likely explanation is that we investigated a different anatomical region and a more specific neuropsychological function. On the other hand, lack of statistical correlation in healthy subjects suggests the possibility that dorsolateral prefrontal cortex neurons identified by low
N-acetylaspartate levels may be critical effectors of the cortical pathophysiology implicated in the working memory deficits of schizophrenia. To the extent that reduced
N-acetylaspartate measures are a reflection of impaired functional integrity of neurons, these dorsolateral prefrontal cortex neurons may constitute a rate-limiting factor for working memory performance. Therefore, this putative impairment may constrain the variance in a predictable way, making the correlation in patients more evident than in healthy subjects. This assumption also is consistent with earlier data that showed a correlation between prefrontal
N-acetylaspartate measures and activation of the distributed working memory cortical network in patients but not in normal subjects
(22). These data were obtained in two groups of patients during performance of the Wisconsin Card Sorting Test and the N-back test. Both data sets showed more pronounced correlations between
N-acetylaspartate levels in the right dorsolateral prefrontal cortex and regional cerebral blood flow during performance of the tasks in patients but not in comparison subjects, once again suggesting that the correlation is more robust in patients. Moreover, the selectivity of the correlation between 2-back working memory performance and
N-acetylaspartate measures in the right dorsolateral prefrontal cortex is consistent with prior functional imaging studies. The spatial component of the N-back task is important, as numbers flash in four different locations. In fact, the cortical network activated with fMRI by our version of the N-back task is typical for spatial working memory tasks involving the left and right dorsolateral prefrontal cortex as well as bilaterally in the parietal cortex
(3,
5). Given that the region that most clearly differentiates between patients and comparison subjects is the right dorsolateral prefrontal cortex
(5), it is not surprising that the correlation is found only on that side.
The physiological role of
N-acetylaspartate in neurons has yet to be fully elucidated. In the mature brain (when glial and neuronal cells are completely differentiated),
N-acetylaspartate is found almost exclusively in neurons and in highest concentrations in pyramidal glutamatergic neurons
(28). A recent in vitro study has also reported that oligodendrocytes express small concentrations of
N-acetylaspartate
(29).
N-Acetylaspartate synthesis takes place in the mitochondria
(30), is ADP dependent
(30), and is tightly coupled to glucose metabolism
(31). The synthetic reaction is a transamination catalyzed by
l-aspartate-
N-acetyl transferase that uses glutamate (source for aspartate) and either pyruvate or 3-hydroxybutyrate (source of Acetyl CoA) as substrates
(30). Moreover,
N-acetylaspartate acts via the glutamatergic NMDA receptor to elevate intracellular calcium
(32); its concentrations are reduced by pharmacological inhibition of mitochondrial energy metabolism; and it correlates highly with the relative reduction of ATP and O
2 consumption
(16). All these findings are consistent with a recent proposal that
N-acetylaspartate serves as an osmolyte used by molecular water pumps to expel metabolic water out of neurons
(33). In this model, it has been tentatively calculated that 1 mol of
N-acetylaspartate is produced for 40 mol of glucose or glucose equivalent oxidized in the brain
(33), once again stressing the relationship between
N-acetylaspartate and glucose metabolism. This basic science literature is paralleled by a series of studies in humans that have demonstrated the reversibility of
N-acetylaspartate reductions, thereby suggesting that
N-acetylaspartate is sensitive to pathological processes affecting the functioning of neurons
(16–
21). Moreover, a recent study in healthy subjects and patients with dementia combining
1H-MRSI and [
18F]fluorodeoxyglucose positron emission tomography has demonstrated a positive linear relationship between cerebral metabolic rates for glucose and
N-acetylaspartate concentrations in cortical gray matter
(20). All these studies suggest that
N-acetylaspartate concentrations may vary according to the metabolic status of neurons, especially pyramidal neurons.
A limitation of the present study is that we did not measure absolute concentrations of
N-acetylaspartate. However, the pattern of reductions and of correlations strongly suggests that
N-acetylaspartate levels are accounting for the changes and the correlations
(34). Another limitation is that we cannot address the gray or white matter origin of these
N-acetylaspartate effects because of lack of tissue segmentation. This is important because a 1.4-ml voxel placed in the cortex certainly contains sulcal CSF (no
N-acetylaspartate) and white matter (lower
N-acetylaspartate than gray matter—as shown by Lim et al.
[35]). Therefore, a systematic difference in voxel placement/selection could result in different tissue composition in the voxels between groups and account for differences in the measured spectra. Also, use of ratios only partially addresses this limitation, since there is evidence that metabolites can vary independently
(36). To try to address the potential confounder addressed by segmentation correction, i.e., biased voxel placement or anatomical variation in tissue compartments across groups, we performed further analyses. CSF, white matter, and gray matter have different T
1 relaxation, thus providing different signal intensities. On the basis of this principle, we reasoned that if the dorsolateral prefrontal cortex and the hippocampal area contained systematically different quantities of CSF, white matter, and gray matter in patients and comparison subjects, some difference in signal intensity in those regions of interest should be measurable between the two groups. Therefore, we superimposed the spectroscopic regions of interest from the dorsolateral prefrontal cortex and the hippocampal area onto the T
1-weighted images and measured the signal intensity. Since the signal intensity can be influenced by several factors in each individual, we then normalized the signal intensity from the region of interest to the signal intensity of the whole MRI slice. Statistical comparison between patients and comparison subjects on the normalized signal intensity from the dorsolateral prefrontal cortex and the hippocampal area did not reveal any significant difference (all t<1.4, all p>0.20). Also, the number of voxels included in regions of interest did not systematically differ between patients and comparison subjects (all t<1, all p>0.20) (
Table 1).
In conclusion, the results of the present study suggest that neuronal pathology in the hippocampal area and the dorsolateral prefrontal cortex may be present even at the onset of schizophrenia and is not the result of chronicity. Moreover, the neuronal changes in the dorsolateral prefrontal cortex monitored with this assay seem to be associated with poor performance on working memory tasks.