Evidence of Maternal Smoking During Pregnancy and ADHD
Results from animal studies indicate that hyperactivity in the offspring may result from prenatal nicotine exposure
(14) and that the effects are long-lasting
(28). Possible mechanisms may be modulation of the dopaminergic system and a greater number of nicotine receptors
(29,
30). Human data
(31–
33) show an association between maternal smoking during pregnancy and low birth weight, preterm delivery, and stillbirth. However, one review
(34) reported inconsistent associations between prenatal smoking and long-term intellectual and developmental abilities.
We identified 24 studies that evaluated the association between smoking during pregnancy and ADHD or ADHD symptoms. Eight studies
(35–
42) used the diagnostic criteria of ADHD as the outcome, and 16
(43–
58) studied ADHD subgroups. Deficit in attention, motor control, and perception
(47) and minimal brain dysfunction
(57) were examined in two of the studies and were included in the review because these diagnoses encompass the core symptoms of ADHD.
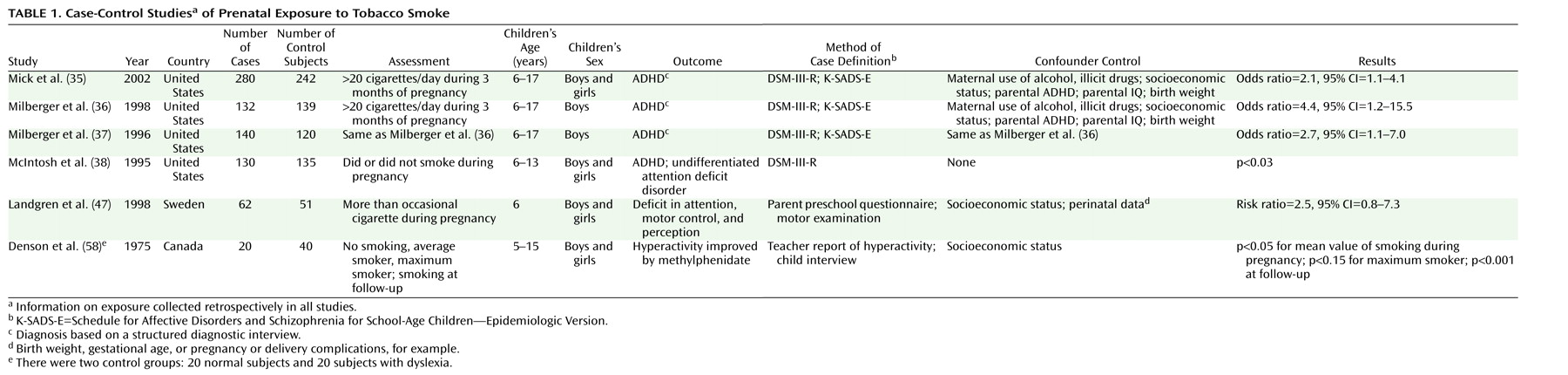
Table 1 lists the main characteristics of the case-control studies, and
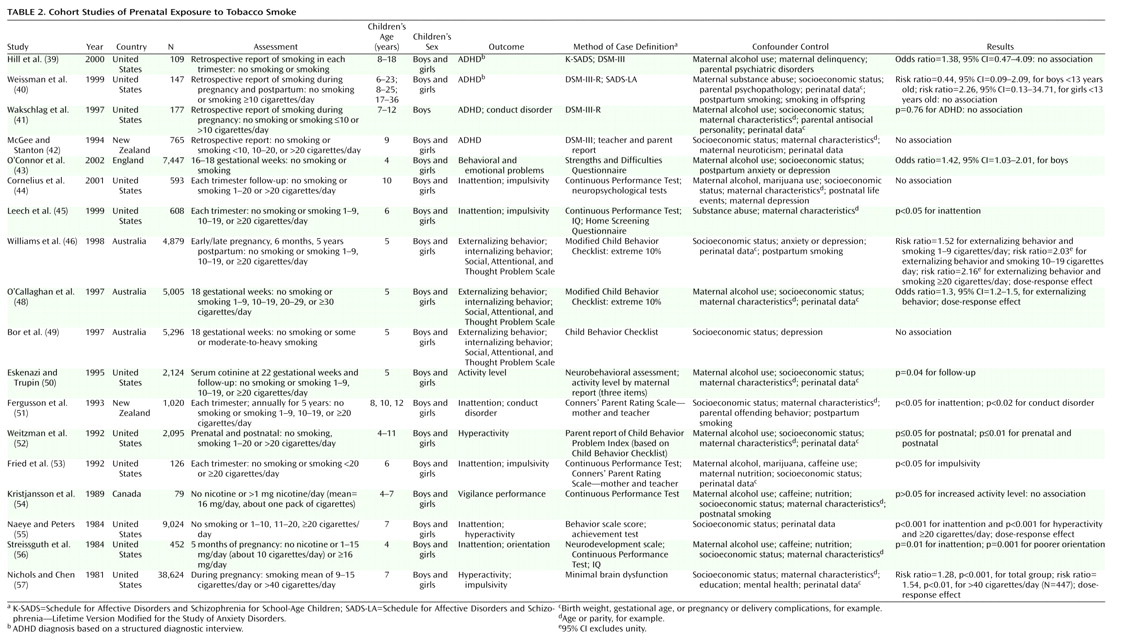
Table 2 lists the main characteristics of the cohort studies.
The case-control studies presented in
Table 1 provide support for the hypothesis that prenatal exposure to nicotine may result in ADHD symptoms. Milberger et al.
(36) attempted to differentiate between genetic vulnerability and prenatal smoking by controlling for parental ADHD and by including siblings of children with ADHD in the model. The results showed that prenatal maternal smoking was associated with a fourfold higher risk of ADHD in the offspring independently of maternal disorder, similar to the risk accounted for by maternal ADHD. Results of the study of Mick et al.
(35), based on the 1996 data of Milberger and co-workers
(36,
37) and supplemented with data for girls, showed a lower odds ratio of 2.1.
Table 2 presents 18 follow-up studies of maternal smoking during pregnancy
(39–
46,
48–57). Four of these studies
(39–
42) classified ADHD according to DSM criteria and collected information on maternal smoking habits during pregnancy retrospectively. Two of the studies
(40,
42) assessed smoking as a dichotomized measure. The children were selected from families with alcoholism
(39), major depression
(40), and other clinical referral symptoms
(41). In contrast, McGee and Stanton
(42) examined a sample of 765 children whose mothers were randomly selected and recruited at the time of the child’s birth. No association between prenatal smoking and ADHD was found in any of these four studies
(39–
42).
Eleven of the follow-up studies assessing only prenatal maternal smoking or both prenatal and postnatal smoking indicated an association between smoking and ADHD or ADHD-related symptoms.
Six cohort studies
(40,
44,
46,
50–52) and one case-control study
(58) aimed at differentiating the effect of in utero and postnatal exposure to smoke in relation to ADHD
(40) and ADHD subgroups
(44,
46,
50–
52,
58). Several studies measured prenatal maternal smoking on an ordinal scale, but one dichotomized groups into no smoking and smoking 10 or more cigarettes per day or less frequently during pregnancy
(40). Only one study used maternal serum cotinine to verify smoking status
(50).
The results from the studies examining differences between prenatal and postnatal smoking varied. In a study by Weissman et al.
(40), postpartum smoking was not associated with ADHD in the offspring. Information on exposure for this study was collected retrospectively (three times over a 10-year period) for a small group ranging widely in age from 6 to 23 years. Similarly, prenatal and postnatal maternal smoking were not found to have any impact on attention deficits or impulsivity in a larger sample of 10-year-olds
(44).
Two other studies on relatively large samples of about 2,000 children
(50,
52) revealed statistically significant associations between postnatal but not prenatal smoking and hyperactivity
(52) and activity level
(50). There was a very low frequency of exclusively prenatal or postnatal smokers in the study by Weitzman and co-workers
(52), which makes it difficult to disentangle the independent contribution made by each variable. Eskenazi and Trupin
(50) primarily designed their study to test cognitive deficits and included only three items on activity level (the child has more energy than most, hates to sit still, and likes to play quietly) rated by the mother. It is unlikely that the selected items were sensitive enough to capture the full concept of hyperactivity.
Statistically significant associations between prenatal smoking and the outcome were reported in two studies concerning externalizing behavior
(46) and inattentive symptoms
(51). The studies collected data prospectively, had large samples, and controlled for maternal postpartum smoking and socioeconomic status
(46,
51). The study by Fergusson and co-workers
(51) used teachers as informants in addition to maternal reports.
Eight of the follow-up investigations are particularly important because of large samples, prospective exposure assessments
(43,
46,
48,
49,
55–57), and appropriate designs with respect to control for potential confounders such as alcohol
(43,
48,
53,
56) and socioeconomic status
(43,
46,
48,
49,
53,
55–57). Moreover, a variety of techniques were employed in these studies to assess symptoms, including observational measures of child behavior. These studies reported small but independent effects of smoking on a variety of symptoms related to ADHD in younger children (4–7-year-olds). Four
(46,
48,
55,
57) of the eight studies found a dose-response-like association.
Alcohol Consumption During Pregnancy and ADHD in Childhood
Disturbed attention and neuromotor development has been found in monkeys prenatally exposed to moderate levels of ethanol
(15). Ethanol enhances migration of nerve cells, which is hypothesized to be involved in behavioral difficulties in childhood. It also interferes with the production of neuroendocrine hormones, which may perturb brain growth
(59).
In humans, high levels of alcohol consumption during pregnancy are associated with a greater risk of congenital malformations
(60) and possibly stillbirth
(61). Alcohol is widely recognized as a teratogenic agent causing CNS dysfunction and impaired mental functioning, including fetal alcohol effect
(62) and fetal alcohol syndrome, which incorporates the core symptoms of ADHD
(63).
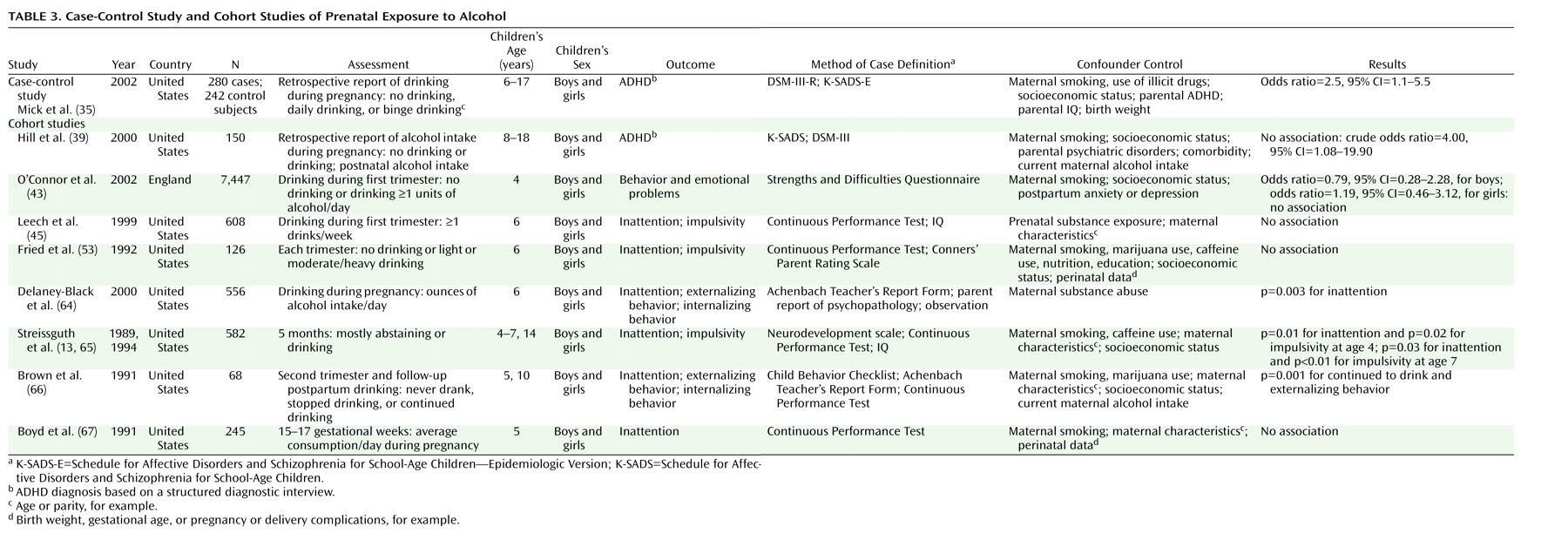
Table 3 presents nine studies investigating the association between prenatal alcohol exposure and ADHD
(35,
39) or ADHD subgroups
(43,
45,
53,
64–67). Alcohol consumption was significantly associated with ADHD
(35) and subgroups of ADHD
(64–
66) in four of these nine studies, two of which involved high levels of alcohol exposure during pregnancy
(35,
65).
A recent case-control study of prenatal exposure to alcohol by Mick and co-workers
(35), which also studied smoking, found that twice as many children with ADHD had mothers who either drank alcohol daily or binged heavily during pregnancy (N=10) than children without ADHD (N=5). However, these results were not supported by Hill and colleagues
(39), who used dichotomized exposure data collected retrospectively. In this cohort study, the univariate association between prenatal alcohol exposure and ADHD disappeared after the authors adjusted for familial risk of alcoholism, intrauterine exposure to smoking, maternal current alcohol intake, or information on alcohol and parental psychopathology. Studies using the Continuous Performance Test
(45,
53,
67) and Conners’ Parent Rating Scale
(53) reported no effect of prenatal exposure to alcohol on inattention or impulsivity.
The Pregnancy and Health Study of Streissguth et al.
(13) is a birth cohort of 582 children exposed to alcohol in utero. Mothers were categorized as abstainers (light/infrequent drinkers or abstainers) or heavy drinkers (one drink or more per day) during pregnancy. The 4–7-year-old children of heavy drinkers were more likely to exhibit attention deficits and impulsivity on the Continuous Performance Test than were children of abstainers. The association remained statistically significant after adjustment for prenatal maternal smoking, prenatal caffeine intake, and socioeconomic status. In the study by Brown et al.
(66), prenatal alcohol exposure was found to be related to externalizing behavior problems, and in the study of Delaney-Black et al.
(64) it was related to attention deficits. The largest cohort study, that of O’Connor et al.
(43), which involved nearly 7,500 children, did not find an association between alcohol exposure and general behavioral problems.
The maternal psychological state may influence the intrauterine environment by altering the uterine blood flow
(72), thereby possibly impeding nutrient supply to the fetus. Maternal stress during pregnancy has been associated with greater prevalence of some congenital malformations
(73) and with changes in fetal levels of cortisol, the main hormone associated with stress
(74), infant attention regulation
(75), schizophrenia
(76), social behavior
(77), and depression
(78).
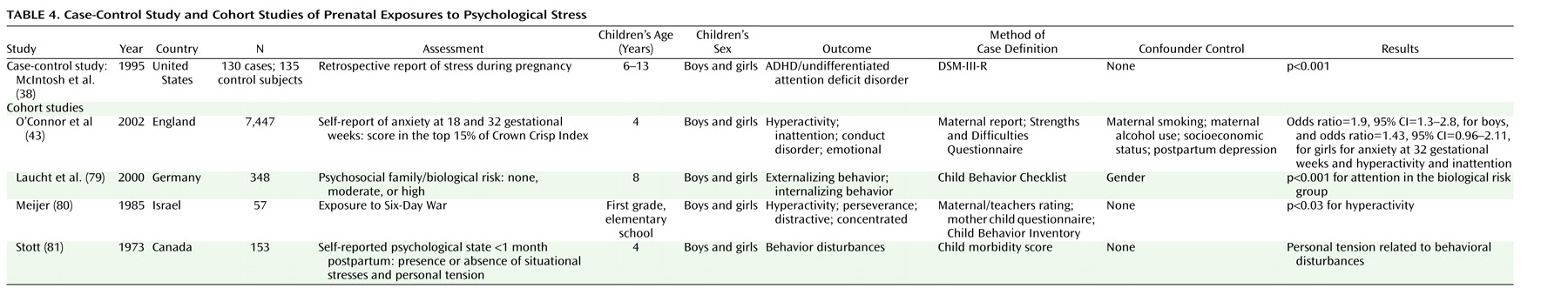
We identified one study on maternal stress during pregnancy and ADHD
(38) and four on ADHD subgroups
(43,
79–81), as shown in
Table 4. Results from the case-control study
(38) showed that mothers of children with ADHD and undifferentiated attention deficit disorder reported psychological stress more frequently during pregnancy. No adjustment for confounding was performed. Thirty years ago, a host of possibly stressful circumstances during pregnancy (measured 1 month postpartum) were found to predict behavioral disturbances in childhood
(81). A more recent study
(79) used the attention scale on the Child Behavior Checklist and found that children of mothers exposed to stressful family circumstances during pregnancy had a higher likelihood of attention problems. The definition of stressful family circumstances was a combination of genetic and psychosocial factors. Meijer
(80) conducted a natural experiment by comparing children of mothers exposed to wartime stress with children born 2 years later. The mothers’ and teachers’ ratings of child behavior problems were higher in the group exposed to war during the early postpartum months rather than during pregnancy. None of these studies assessed the amount of stress experienced during pregnancy with a validated instrument, nor did they control for confounding.
Recently, a large community-based study assessing psychosocial stress during pregnancy was conducted in England
(43). After controlling for maternal smoking, alcohol intake (dichotomized variables), and postpartum depression, the authors found that maternal anxiety at week 32, but not at week 18, was associated with hyperactivity and inattention in boys. The results were statistically significant, not only when the upper extreme of the behavioral problem distribution was examined but also when behavioral problems were examined as a continuous variable. Women with high scores on anxiety at gestational week 18 were more likely to drop out of the study. There was no control for parental psychopathology. Furthermore, a high correlation between anxiety scores measured during and after pregnancy makes it difficult to exclude the possibility that the association was due to genetics rather than anxiety in the mother.