Understanding the anatomy and function of motivational brain systems may provide important information about correspondences between impulsivity, risk for substance use disorders, and adolescence. Motivation can be conceptualized as brain activity that processes “input” information about the internal state of the individual and external environment and determines behavioral “output”
(36). Rather than operating as a simple reflex system producing discrete behaviors in response to discrete stimuli, motivation involves higher-order processing designed to organize behavior to maximize survival
(37). Goal-directed behavior involves integrating information about multiple changing internal states (e.g., hunger, sexual desire, or pain) and environmental conditions (including resource or reproductive opportunities, the presence of danger) in generating an advantageous behavioral response
(31). Compounding this complexity, multiple survival goals may be simultaneously important but independently attainable in space and time, and there may exist large numbers of potentially successful behavioral strategies to attain one or more of these goals. Motivational neurocircuitry should therefore involve mechanisms capable of representing alternative motivated drives and efficiently prioritizing and selecting appropriate motivated drives for enactment
(36,
38).
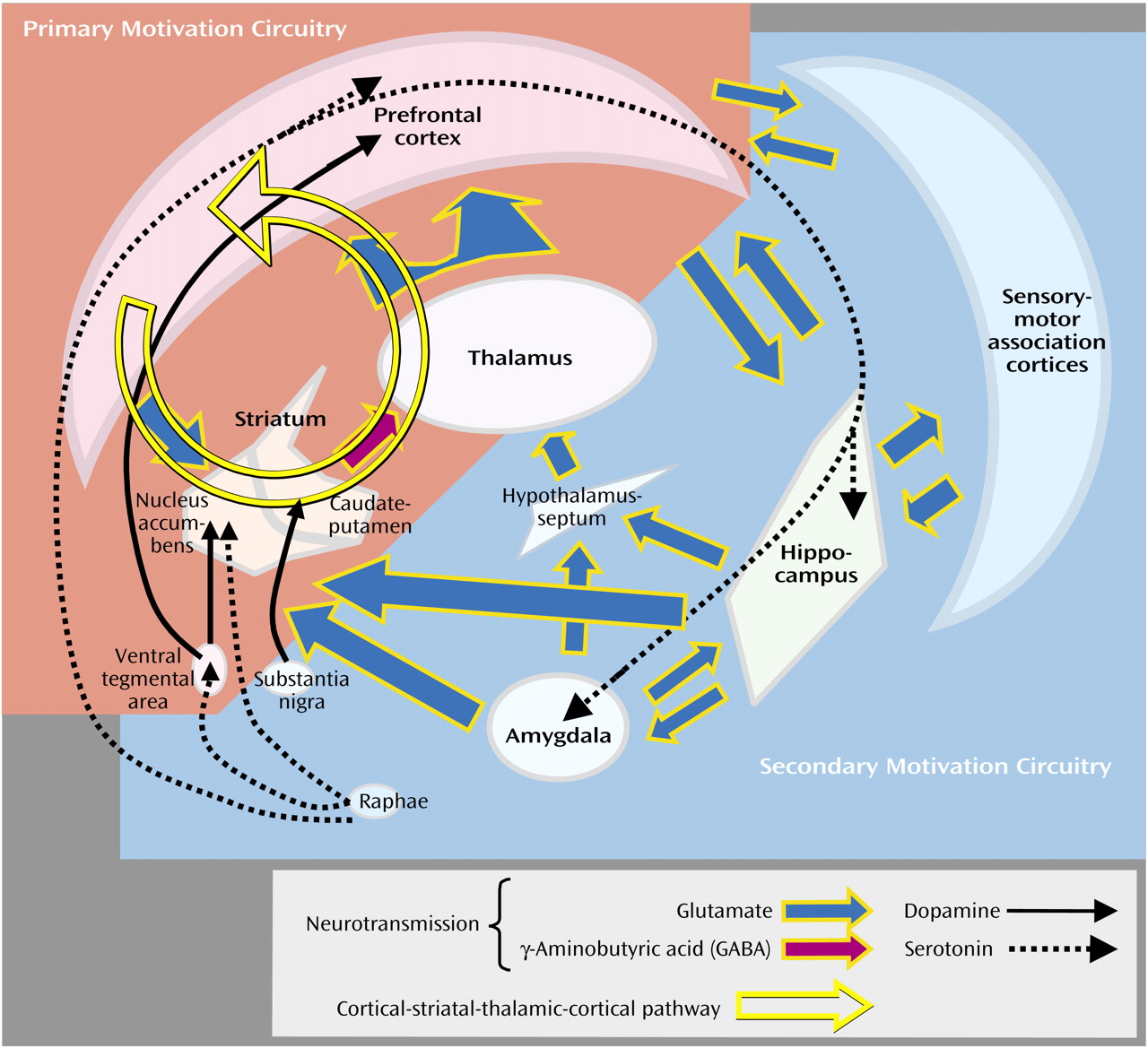
Translational neuroscience is beginning to generate neurobiological evidence supporting these theoretical considerations. The importance of motivation to evolutionary fitness would predict that substantial portions of the brain are involved, following a hierarchical anatomical and functional organization conserved across species. Animal and human studies suggest the existence of a primary motivation circuitry involving the prefrontal cortex and ventral striatum, which has direct access to and influence on motor “output” structures
(37). This anterior system is supported by a more widely distributed and posteriorly situated secondary motivation circuitry that provides multiple modalities of sensory “input” information by means of direct axonal projections converging into primary motivation circuits (
Figure 1)
(39–
41). For example, the hippocampus and amygdala provide contextual memory and affective information relevant to motivational stimuli
(31,
39,
42,
43), while hypothalamic and septal nuclei provide information relevant to primitive or instinctual motivated behaviors, such as nutrient ingestion, aggression, and reproductive responses
(44).
Recent findings characterize primary motivation circuitry as containing populations of neurons capable of generating firing patterns that may encode multiple aspects of motivated drives or alternative motivated drives
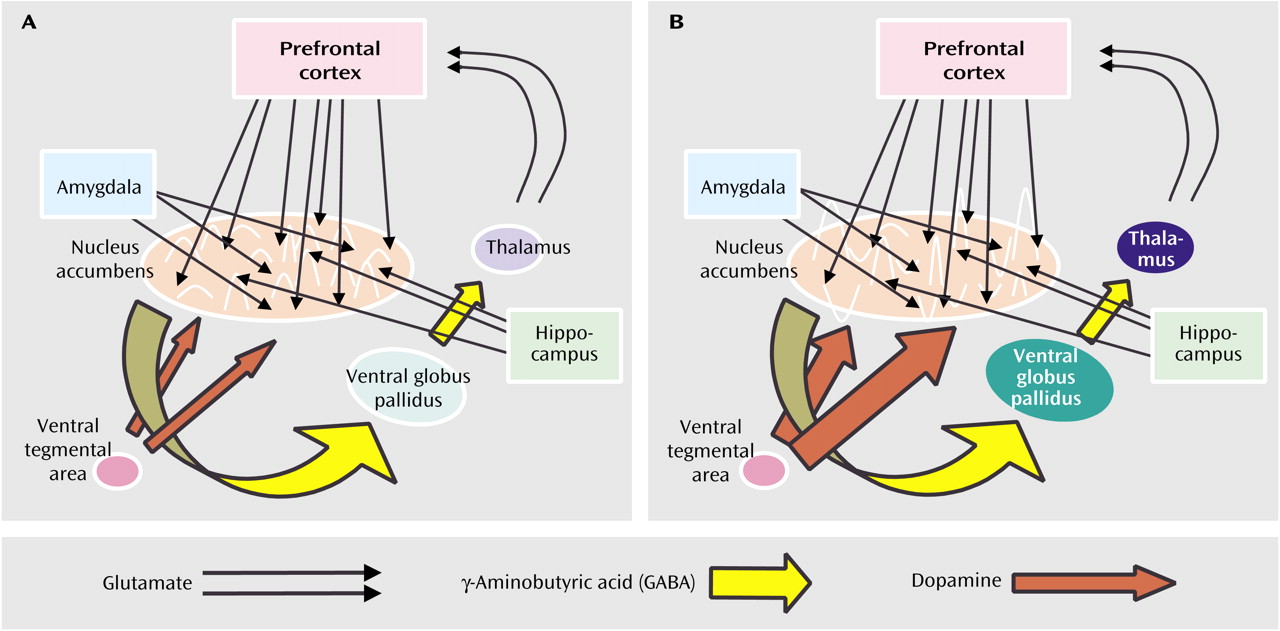
(45). These representations occur among neuronal ensembles interconnected by parallel loops of serial axonal projections from the prefrontal cortex to the ventral striatum (the nucleus accumbens to the ventral globus pallidus) to the thalamus and back to the cortex
(46,
47) (
Figure 1 and
Figure 2). Cortical-striatal-thalamic-cortical loops are described as parallel because specific subregions of the prefrontal cortex (e.g., anterior cingulate, ventromedial, and dorsolateral regions) project to specific compartments within the striatum, which in turn maintain some degree of segregation in projections to the thalamus and back to the cortex
(48). Both anatomical and neurophysiological evidence suggest that firing patterns of neuronal ensembles within functionally specific compartments of the striatum are in part correlated with patterns of firing in specific prefrontal cortex subregions
(42,
49). In turn, firing patterns in both the nucleus accumbens and prefrontal cortex are influenced by glutamatergic inputs from the hippocampus and amygdala, suggesting that abnormalities in these distal structures may produce both mental illness and motivational disorders
(50). Because striatal populations have direct influence on premotor and motor cortices and brainstem motor centers, their activity more directly determines motivational states and behavioral output
(39,
44). Dense collections of γ-aminobutyric acid (GABA)-ergic inhibitory neurons in the striatum communicate by means of recurrent collateral inhibition that is suggestive of the high capacity of local neural networks to encode vast numbers of alternative firing patterns that could serve as computational building blocks of multiple, highly elaborated motivated drives
(39,
47,
51–67).
Accumulating evidence suggests that neurocircuitry encoding repertoires of alternative motivated drives are subject to neurobiological events that prioritize and select motivated drives for behavioral action. Particular neural substrates have been associated with promoting (increasing the probability of enactment) or inhibiting motivated drives. Disturbances of motivational repertoire, including varieties of impulsivity and addictions, may thus commonly reflect poor coordination or abnormal functioning of promotional or inhibitory neural systems integral to primary motivation circuitry
(41,
52). Consistent with this notion, neuroimaging studies implicate common subcortical-striatal regions and the prefrontal cortex in emotional and cognitive processes of decision making and the pharmacological action of addictive drugs
(53). To further explore this hypothesis, data characterizing promotional and inhibitory motivation substrates will be described, followed by a review of changes within these pathways during adolescence.
Promotional Motivation Substrates
Dopamine release into the striatum is a principal neuromodulatory event implicated in the translation of encoded motivated drives into action, operating like a general “go” signal
(54). Dopamine release into the ventral striatum (nucleus accumbens) and dorsal striatum (caudate putamen) is provoked by excitatory signals from the cortex and other areas that stimulate dopamine neuron activity in the ventral tegmental area and substantia nigra, respectively
(55,
56) (
Figure 1). However, the ventral and dorsal sections are associated with different levels of premotor processing. Dopamine release into the dorsal striatum, compromised in the pathogenesis of Parkinson’s disease, is primarily associated with the initiation and flow of concrete motor activity and habitual behavior
(57). In contrast, dopamine release into the nucleus accumbens is associated with motivational stimuli, subjective reward, premotor cognition (thought), and learning of new behaviors
(43,
46,
58). The precise manner in which dopamine release is involved in the translation of thought into action is unknown. Some work indicates that dopamine discharge directly affects the firing patterns of neuronal ensembles in the nucleus accumbens and influences their responses to glutamatergic input from the cortex, amygdala, and hippocampus
(51,
59) (
Figure 1B). This finding suggests that sensory, affective, and contextual memory information, leading to the generation of representations of motivated drives, is gated by dopamine release in the striatum, such that downstream motor centers can receive and act upon specific motivational information
(51,
59,
60). Accordingly, neurotoxic lesions of the prefrontal cortex, amygdala, or hippocampus alter behavioral repertoires provoked by pharmacological stimulation of dopamine release in the nucleus accumbens
(61–
63).
A wide variety of motivational stimuli have been shown to increase dopamine in the nucleus accumbens. These include the pharmacological actions of addictive drugs (including nicotine, alcohol, cocaine, amphetamine, opiates, cannabis), natural rewards (food, sex, or other resources), reward-related stimuli and situations (video-game playing), and stressful or aversive stimuli
(43,
64–67). Environmental awareness is vital for the efficient acquisition of reward resources, and the drive to seek and explore the unknown is itself a powerful primary motivation
(43). Environmental novelty provokes ventral-striatal dopamine release
(68) and, like addictive drugs, produces locomotor behavior in laboratory animals
(69). Novelty, presented in the form of unpredicted contingencies or environmental stimuli, in combination with addictive drugs, is particularly motivating
(70). Rewards delivered in intermittent, random, or unexpected fashions have greater capacity over repeated trials to maintain dopamine cell firing and reward-conditioned behavior
(71,
72). In contrast, many well-learned motivated behaviors or habits performed under expected contingencies become less dependent on nucleus accumbens dopamine release. Thus, direct pharmacological stimulation of dopamine systems mediated by addictive drugs appears to mimic and/or act synergistically with the natural motivational-encoding properties of environmental novelty.
A second important function of dopamine, together with glutamatergic afferent activity in the nucleus accumbens and intrinsic GABA-ergic activity of nucleus accumbens neurons, involves the determination of future representations and selection preferences of motivated drives. In reward-related learning, future behavior is shaped according to past experiences associated with rewards by means of neuroplastic changes in nucleus accumbens neurons
(73). Repeated drug-provoked dopamine release in the nucleus accumbens induces changes in cellular proteins involved in intracellular receptor signaling pathways, gene expression, and cellular architecture
(15). Dopamine transmission in nucleus accumbens and prefrontal cortex regions projecting to the nucleus accumbens has been implicated in mechanisms of learning and plasticity, including changes in long-term potentiation and morphology of neuronal dendritic trees
(74–
77). These neuroplastic processes may underlie behavioral sensitization, whereby the motivational drive associated with a reward becomes increasingly stronger as that reward context is repeatedly experienced
(78,
79). Sensitization, as an increase in the motivational priority associated with a particular contextual reward relative to other encoded motivational drives, produces reward-specific acquisition behavior that becomes increasingly compulsive
(78). In this manner, dopamine systems activity may serve a long-term function of narrowing or focusing the repertoire of motivational drives of the individual.
Inhibitory Motivation Substrates
Deficiencies of function or structure of inhibitory systems are associated with the enactment of motivated drives deemed suboptimal or inappropriate. Chief among these are the serotonin (5-HT) neurotransmitter system and prefrontal cortex components of motivational circuitry (
Figure 1). Measures of decreased brain 5-HT activity are associated with impulsive behaviors, including outward and self-directed violence, suicide, fire starting, and pathological gambling
(80–
82). Pharmacological injury of 5-HT systems in animals results in impulsive responding in reward-related learning and incentive motivation
(83). Conversely, pro-serotonergic agents decrease social aggression and impulsivity in animals and humans
(84,
85). Although mechanisms for these findings have not been fully elaborated, 5-HT projections from the midbrain raphae nuclei to motivational circuitry, including the ventral tegmental area, nucleus accumbens, prefrontal cortex, amygdala, and hippocampus, appear involved
(55,
86).
Prefrontal cortex function has long been associated with impulse control. Documented as early as 1848, damage to the ventromedial prefrontal cortex causes pervasive motivational impulsivity associated with affective instability, poor decision making and executive planning, and indifference to social cues
(87). Impaired impulse control has subsequently been reported in numerous neuropsychiatric conditions (e.g., antisocial personality disorder, affective disorders, schizophrenia, substance use disorders, dementias, and traumatic brain injury) characterized by abnormal measures of prefrontal cortex function
(26,
30,
88–90).
Prefrontal cortex abnormalities are associated with a greater risk of developing substance use disorders, possibly involving changes in motivational responses to addictive drugs. Clinical studies demonstrate an association of traumatic brain injury, often involving the prefrontal cortex, with heightened substance use disorder comorbidity and suggest that the onset of either of these factors alone increases risk for the other
(91–
93). Functional or anatomical abnormalities of the prefrontal cortex of nonspecific etiology are also commonly identified in populations with substance use disorders
(94–
97). Corresponding to these clinical observations, prefrontal cortex lesions in rats can augment the reinforcing efficacy of cocaine during self-administration
(98,
99).
Investigations of corticostriatal interactions suggest a mechanism for prefrontal cortex dysfunction, producing both impulsivity and greater risk for substance use disorders. Excitatory glutamatergic projections from the prefrontal cortex to the nucleus accumbens and ventral tegmental area influence dopamine release, neuronal firing, and neuroplastic processes in the nucleus accumbens
(39,
100,
101). These anatomical and functional linkages suggest that the prefrontal cortex is involved in the representation, execution, and inhibition of motivational drives by influencing patterns of neural ensemble firing in the nucleus accumbens. Compromise of the prefrontal cortex or its inputs to the nucleus accumbens could 1) alter the variety of representations of motivational drive options in the nucleus accumbens, 2) alter the response patterns across nucleus accumbens neuronal ensembles to the “go” signal provided by dopamine influx, resulting in greater probability of enactment of particular motivated drives, and/or 3) impair neuroplastic processes in the nucleus accumbens that would normally decrease the strength of motivated drives deemed inappropriate by prior experience. Poor prefrontal cortex function, regardless of the specific pathology, could increase the probability of performing inappropriate motivated drives viewed clinically as impulsive. Similarly, prefrontal cortex dysfunction may result in 1) preferential motivational responding to directly encoded rewards provided by the prodopamine effects of drugs and/or 2) an unchecked progression of neuroadaptive effects of drugs underlying motivational sensitization and a switch to compulsive drug seeking
(102,
103). As such, relative impairment of inhibitory motivational systems in the setting of robust promotional motivation systems activity would commonly increase impulsivity and the risk of substance use disorders. Neurodevelopmental changes during adolescence leading to these conditions could generate heightened addiction vulnerability.