Panic disorder is a disabling psychiatric condition associated with significant impairment in psychosocial and occupational functioning. Epidemiological studies have consistently estimated lifetime prevalence rates at 2%–4%
(1,
2). Panic disorder is frequently accompanied by other psychiatric disorders, notably depression; 55.6% of those with panic disorder also meet lifetime criteria for major depression
(3). In recent years, particularly within the last decade, the pharmacological treatment of panic disorder has changed as different treatment options have been developed. The differing efficacy and safety of these agents presents the challenge of choosing the best medication for those diagnosed with panic disorder.
Historically, panic disorder was first treated with tricyclic antidepressants and monoamine oxidase inhibitors (MAOIs). However, tricyclic antidepressants and MAOIs carry many intolerable side effects, from anticholinergic and adverse cardiovascular effects in tricyclic antidepressants to strict dietary restrictions and overall poor tolerability in MAOIs
(4). These side effects are thought to decrease overall patient compliance. Several safety concerns also exist with tricyclic antidepressants and MAOIs. For example, the therapeutic margin of tricyclic antidepressants is low; the lethal dose is approximately three times the maximum therapeutic dose. For MAOIs, a hypertensive crisis can occur from the ingestion of foods containing high levels of tyramine, which can be lethal.
Benzodiazepines have been used as a treatment for panic disorder for the past 15 years. They have an advantage over MAOIs and tricyclic antidepressants in their rapid onset of action in comparison to the relatively slow onset of MAOIs and tricyclic antidepressants. However, the long-term use of benzodiazepines may lead to dependency and withdrawal if they are not tapered properly, and some patients who use benzodiazepines find their sedative side effect to be adverse. Benzodiazepines as a single agent are also generally not effective in treating comorbid major depression
(5,
6) and also carry a potentially lethal danger in overdose when combined with alcohol.
Currently, selective serotonin reuptake inhibitors (SSRIs) are indicated as the first-line treatment for panic disorder
(8). The APA’s
Practice Guideline for the Treatment of Patients With Panic Disorder states that SSRIs are likely to have the most favorable balance of efficacy and adverse effects
(7). SSRIs have an advantage over other treatment agents in that they are safer in overdose and have a significantly lower rate of adverse events, particularly fewer cardiovascular symptoms, than tricyclic antidepressants and no dietary restrictions, as do MAOIs. When compared with benzodiazepines, SSRIs carry a lower risk of causing physiological dependence, although there are studies that have found a discontinuation syndrome upon abrupt discontinuation of SSRIs
(9,
10), which is associated with dizziness, nausea, lethargy, and headaches. Finally, the use of SSRIs may be advantageous because of their ease of administration by primary care providers and their ability to effectively treat comorbid disorders (depression, obsessive-compulsive disorder [OCD], social anxiety disorder) that commonly accompany panic disorder
(7).
The purpose of this study was to examine the long-term use of psychotropic medications in patients with panic disorder who were enrolled in the Harvard/Brown Anxiety Research Project. Specifically, the use of SSRIs and benzodiazepines was examined over 10 years to determine if prescribing patterns shifted during the past decade because of the availability of newer pharmacological agents, as well as revised treatment guidelines for panic disorder. Pharmacological treatment patterns were also examined in patients with panic disorder and other comorbid disorders.
Method
The Harvard/Brown Anxiety Research Project is a prospective, naturalistic, longitudinal, multicenter study of adults with a current or past history of anxiety disorders. A total of 711 subjects entered this study from more than 30 clinicians’ practices at 11 different clinical treatment facilities in the New England area. The methods are described in detail elsewhere
(11). Inclusion criteria for participation included a past or current diagnosis of the following at intake: panic disorder with or without agoraphobia, agoraphobia without panic disorder, social phobia, or generalized anxiety disorder. Insufficient for inclusion, but frequently seen as comorbid conditions, were diagnoses of simple phobia, posttraumatic stress disorder, OCD, or anxiety disorder not otherwise specified. Participants must have been at least 18 years of age at intake, have been willing to voluntarily participate in the study, and have signed a written consent form. Exclusion criteria consisted of the presence of an organic brain syndrome and a history of schizophrenia or current psychosis at intake; otherwise, any comorbidity was allowed. A total of 443 patients at intake met the criteria for panic disorder with or without agoraphobia.
The present data derive from the structured diagnostic interview administered at intake (1989–1991) and subsequent semiannual and annual follow-up interviews over 10 years (1991–2001). The initial comprehensive evaluation assessed lifetime history with the Structured Clinical Interview for DSM-III-R Non-Affective Disorders, Patient Version (SCID), and the Research Diagnostic Criteria (RDC) Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L)
(12). Items on the SCID and SADS-L were combined to create the Scalup, a structured interview used to assess diagnoses at intake (available from M.B.K. upon request). The instrument yielded both present and past RDC diagnoses for affective disorders and DSM-III-R diagnoses for nonaffective (including anxiety) disorders. Interviews, conducted by trained research assistants, usually took place in single sessions lasting 2–4 hours. Follow-up evaluations were conducted at 6-month intervals for the first 2 years, annually for years 3–7, and semiannually for years 8–10 with the Upjohn version of the Longitudinal Interval Follow-Up Evaluation
(13). The evaluation gathered weekly information about the presence of specific symptom criteria, psychiatric comorbidity, and psychosocial functioning. Additionally, information regarding pharmacological treatment from the patients was collected every 6–12 months, retrospectively, for each week during the interval and included medication type, average daily dose, and whether patients were taking it as needed.
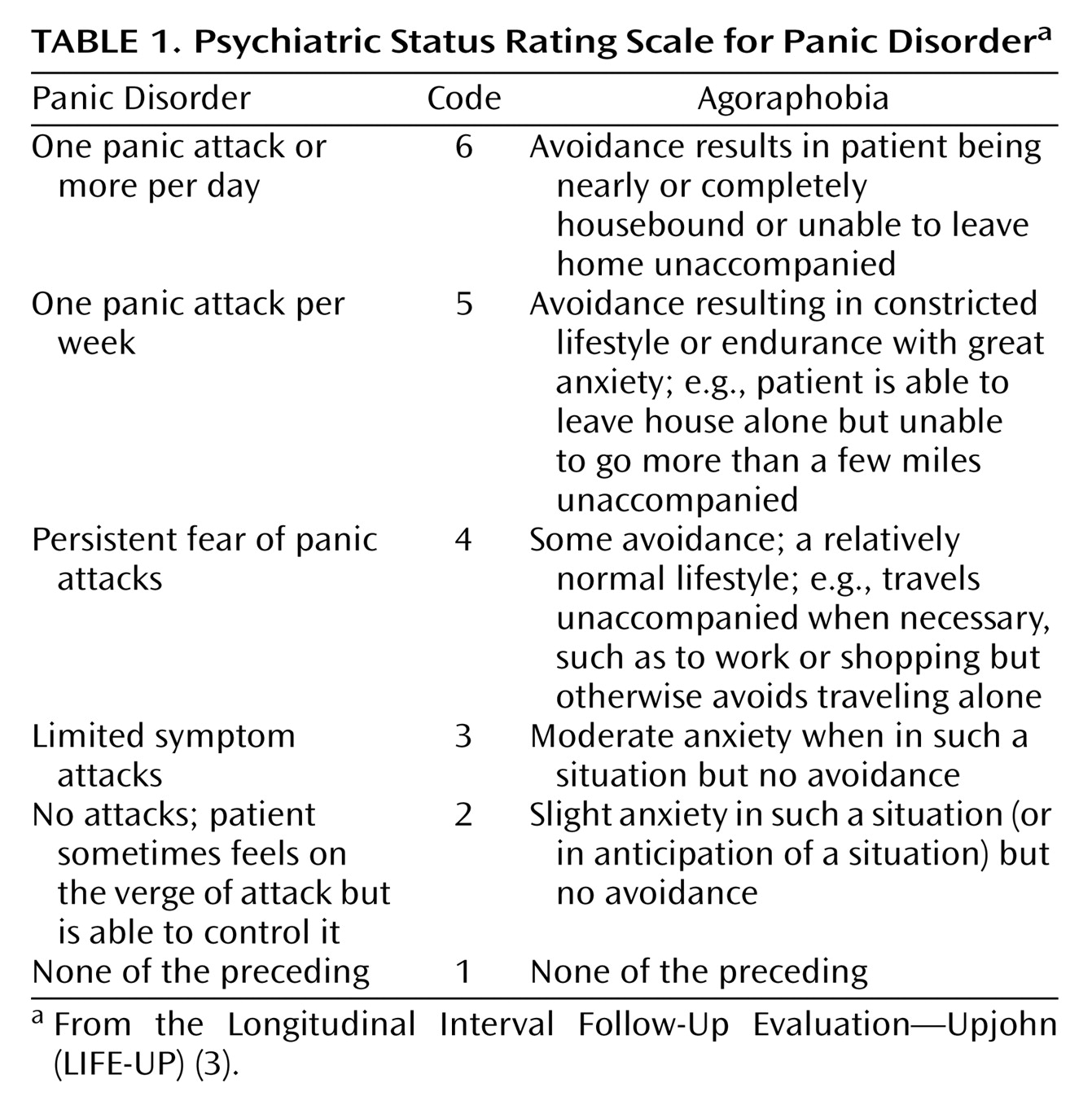
The Longitudinal Interval Follow-Up Evaluation assesses psychopathology with a 6-point psychiatric status rating scale that is scored for each week of the follow-up interval. Psychiatric status ratings for panic disorder and agoraphobia are presented in
Table 1. For example, a psychiatric status rating of 6, representing the greatest severity of illness, requires meeting the full DSM-III-R criteria in addition to having severely disrupted psychosocial and/or occupational functioning. A psychiatric status rating of 5 for panic disorder requires meeting the full DSM-III-R criteria and having at least one panic attack per week. For agoraphobia, a psychiatric status rating of 5 includes active avoidance, resulting in a constricted lifestyle or endurance with great anxiety (e.g., able to leave house alone but not able to go more than a few miles unaccompanied). A participant was considered to have remitted panic disorder if he/she experienced 8 consecutive weeks of psychiatric status ratings of 2 or fewer. This definition of remission has been widely used in studies of affective and other disorders. Additionally, patients with remission from panic disorder were judged to have relapsed if their psychiatric status rating increased to a score of 5 or 6 for 2 consecutive weeks.
Three studies have been conducted with participants already enrolled in Harvard/Brown Anxiety Research Project to assess interrater reliability, subject recall, and validity of psychiatric status ratings from the Longitudinal Interval Follow-Up Evaluation used to assess the course of all disorders
(14). Assessment of interrater reliability of the psychiatric status ratings for anxiety disorder and the other instruments found good-to-excellent reliability. The long-term test-retest study conducted to assess the reliability of using subjects’ retrospective recall to assess psychiatric status ratings over 1 year found very good to excellent reliability for anxiety disorders and major depressive disorder. A separate external validity assessment comparing psychiatric status ratings with other psychosocial measures found good concurrent and discriminant validity.
Statistical analyses were conducted by using SAS version 6.07
(15) and the procedures PROC FREQ, PROC MEANS, and PROC T-TEST. Additionally, proportional hazards regressions (PROC PHREG) with time-varying covariates were conducted to examine significant predictors of SSRI and benzodiazepine use over the 10-year follow-up period. These analyses allowed us to examine how variations within a predictor variable over time influenced the use of an SSRI or benzodiazepine. For example, for the participants who started using an SSRI during our period of observation, we designated the month the use started for that individual as 0. Ratings were then assembled relative to that time, from 1 year before use of that SSRI until 1 year afterward. Proportional hazards regressions were also conducted for the patients whose illness was in remission from panic disorder to examine if receiving various types of psychotropic treatment (SSRIs, benzodiazepines, monotherapy, combined pharmacological treatment) increased the likelihood of remission.
Results
For the analyses in this article, our study group consisted of 443 patients with panic disorder (with or without agoraphobia) who had at least 6 months of follow-up. An additional 44 subjects had a new onset of panic disorder (with or without agoraphobia) during subsequent follow-up periods and were included in these follow-up analyses. Inspection of demographic characteristics of the 443 subjects at intake indicated that the majority of the patients were Caucasian (89%) and female (68%) and had a high level of education (38% had a college education or higher). Slightly more than one-half were married at intake, whereas 27% of the participants reported having never been married. No significant differences were found regarding demographic characteristics between the patients with panic disorder and agoraphobia and those with panic disorder without agoraphobia. Additionally, no significantly different demographic characteristics were found between the patients with panic disorder at intake and those who experienced subsequent onset at some point during follow-up.
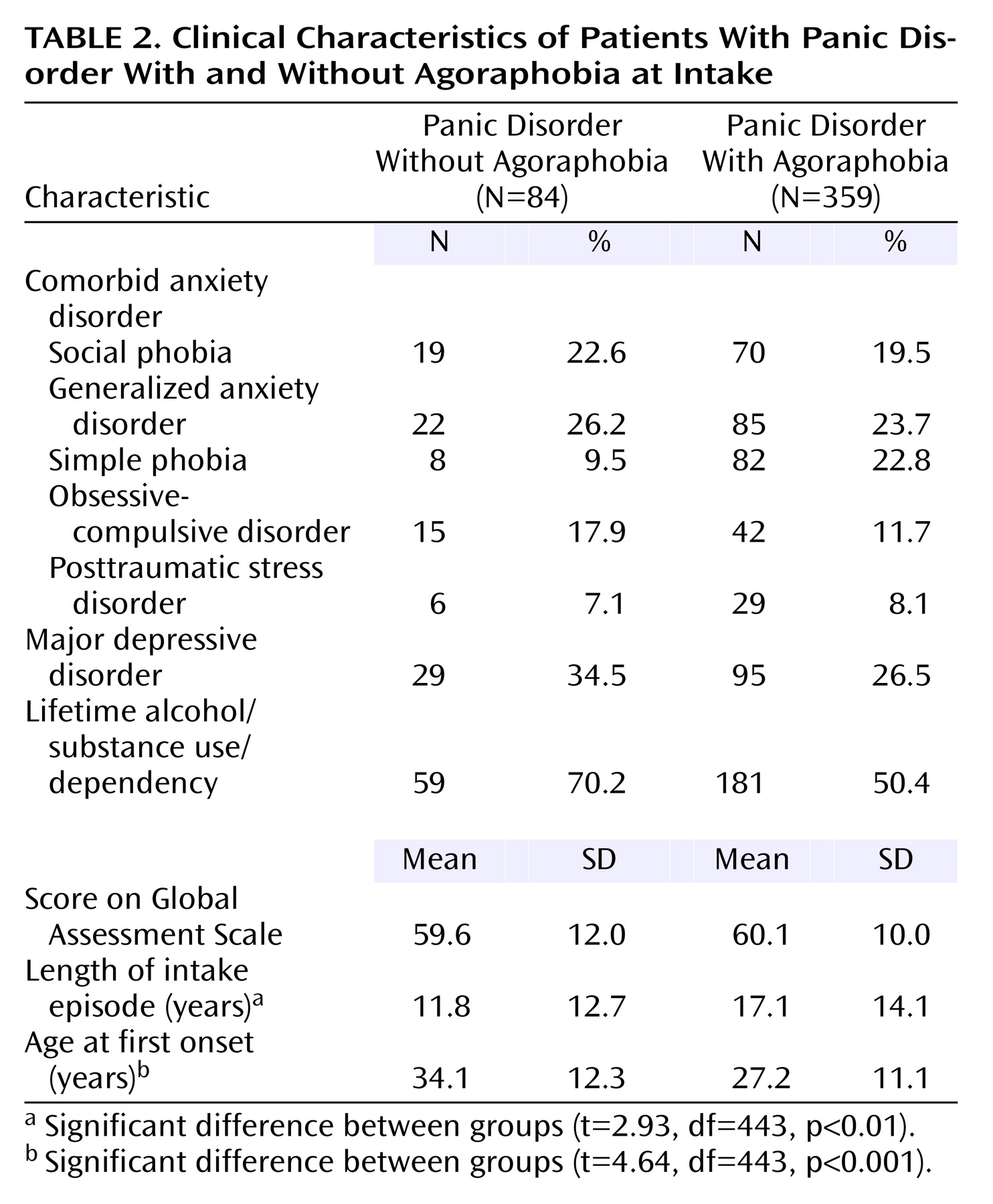
An examination of clinical features showed a high level of comorbidity at the time of initial assessment in the patients with panic disorder (
Table 2). The most commonly occurring comorbid condition was major depressive disorder (28.0%), followed by generalized anxiety disorder (24.2%), and social phobia (20.1%). A total of 54.2% of the patients also met criteria for a lifetime history of alcohol or substance use/dependence. The age at onset of panic disorder was significantly earlier in the patients with agoraphobia (mean=27.2 years) than in those without agoraphobia (mean=34.1 years). Additionally, the length of the intake episode was significantly longer in the patients with panic disorder with agoraphobia (mean=17.1 years) than in those without agoraphobia (mean=11.8 years). No other significant differences were found across clinical features.
Medication Doses
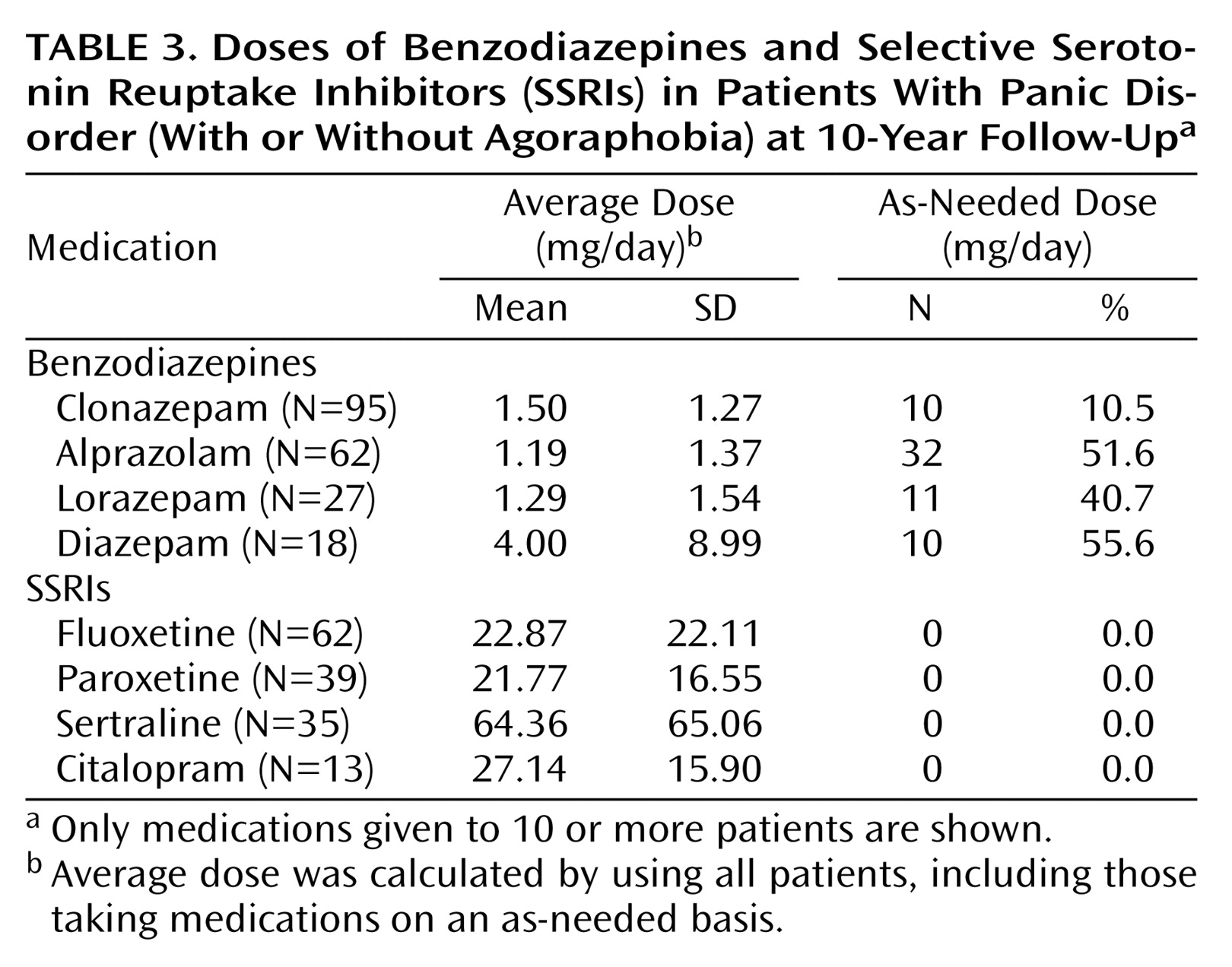
Average doses of specific SSRIs and benzodiazepines are reported in
Table 3. The average daily dose at the 10-year follow-up is reported since some of the medications were not yet developed or approved at intake or in earlier follow-up years. Additionally, the proportion of patients taking benzodiazepines on an as-needed basis is reported. The average daily dose of benzodiazepines was within the lower range that is typically prescribed, although there were large standard deviations in dose because some of the patients were taking extremely low or extremely high doses. Approximately one-half of the patients taking a benzodiazepine were taking it as needed, with the exception of clonazepam (10.5%). For the SSRIs, the average doses were also in the lower typical range. Most of the patients reported taking fluoxetine, followed by paroxetine and sertraline. The patients who were taking a benzodiazepine on an as-needed basis were no more likely to be taking a daily SSRI than were those who were taking a daily benzodiazepine.
Use of Pharmacological Agents Over Follow-Up
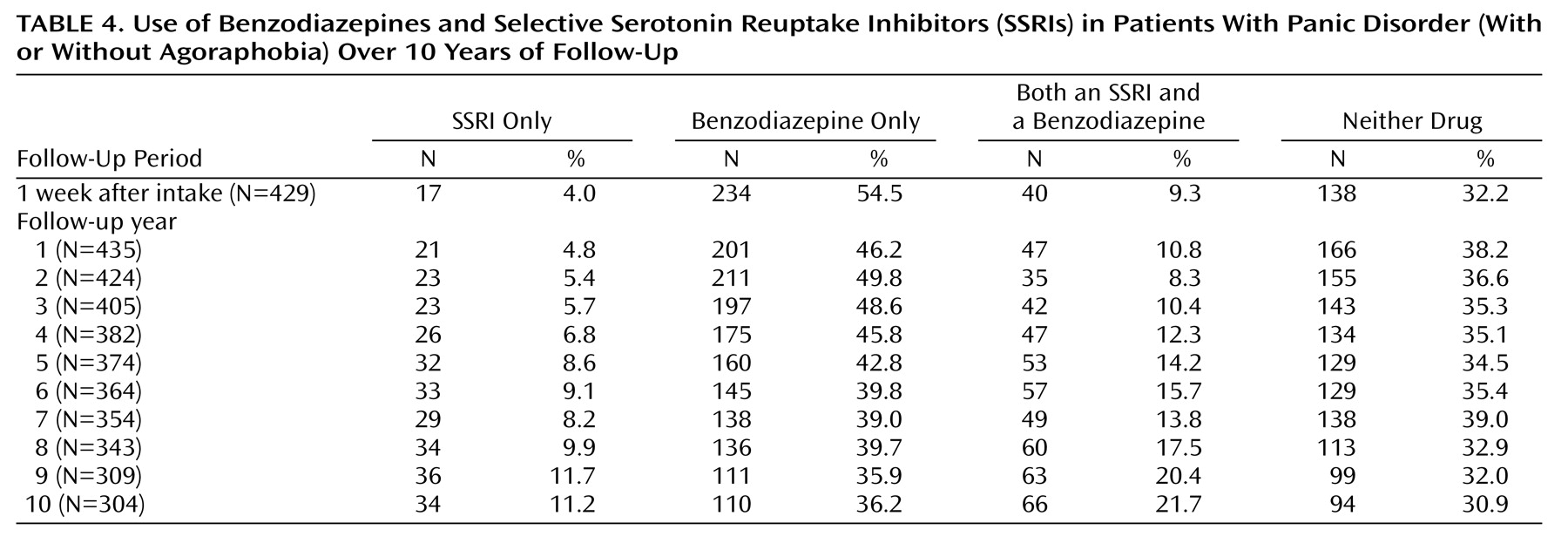
Ten-year use of SSRIs and benzodiazepines in patients with panic disorder is reported in
Table 4. The results indicated a slight decline in benzodiazepine use across the follow-up interval, although overall use of benzodiazepines has remained high, with over one-half of the patients reporting use of a benzodiazepine during each of the follow-up years. A moderate incremental increase in SSRI use was found over time in the patients with panic disorder, from 13.3% at intake to 32.9% at the 10-year follow-up. However, of the patients taking an SSRI, approximately two-thirds were also taking a benzodiazepine in combination during each of the follow-up intervals; 30.9%–39.0% of the patients reported receiving neither drug class during the 10-year follow-up. No significant differences in use of medication were found between the patients with panic disorder without agoraphobia and the patients with panic disorder with agoraphobia.
Because some subjects may have been in remission from their panic disorder during follow-up, a separate examination of medication use was conducted on the patients who were experiencing current episodes (psychiatric status rating of 5 or higher) of panic disorder during each follow-up period. Comparisons of the patients who were having panic attacks at follow-up with the patients who were in remission indicated that experiencing a panic attack did not increase the overall rate of receiving either an SSRI or a benzodiazepine (use at 10 years: 33.7%, 66 of 196, and 62.2%, 122 of 196, respectively).
Next, analyses were conducted to examine the effect of comorbid major depressive disorder on the patterns of psychotropic prescriptions. Our findings showed that the presence of major depressive disorder slightly increased the overall percent of patients receiving an SSRI, although not significantly. Only one-quarter to one-third of the patients with comorbid major depressive disorder reported receiving an SSRI. Of interest, the use of benzodiazepines in the patients with comorbid major depressive disorder also increased. For example, 72.1% (N=44) of the patients with panic disorder and comorbid major depressive disorder reported taking a benzodiazepine at the 10-year follow-up compared with 61.5% (N=83) of the nondepressed patients with panic disorder.
Proportional hazards regressions with time-varying covariates were conducted to examine predictors of SSRI and benzodiazepine use in the patients with panic disorder. The results indicated that comorbid major depressive disorder was a significant predictor of SSRI use during follow-up. The patients with panic disorder and comorbid major depressive disorder were 3.5 times more likely to use an SSRI than those without major depressive disorder (risk ratio=3.6, p<0.0001). Additionally, previous use of a benzodiazepine significantly reduced the likelihood that a patient would receive an SSRI during future follow-ups nearly fourfold (risk ratio=0.3, p<0.0001). Other comorbid conditions, such as alcohol/substance use disorders and additional comorbid anxiety disorders, did not significantly predict SSRI use in patients. No significant predictors of benzodiazepine use were found.
Pharmacological Treatment and Clinical Course
Proportional hazards regressions analyses were conducted to determine if types of psychotropic treatment improved rates of remission in patients with panic disorder. Overall, the patients whose illness remitted were no more likely to be taking an SSRI the week before remission nor were they more likely to be receiving a benzodiazepine the week before remission. Moreover, results indicated that patients who were in remission were no more likely to be receiving combination treatment (SSRIs and benzodiazepines) the week before remission. Finally, analyses examining the intensity of treatment, including dose and whether patients were taking a medication as needed, were conducted to see if there were any effects on clinical outcome. Again, the patients whose disorder remitted were no more likely to be receiving higher doses of benzodiazepines or SSRIs or to be taking a benzodiazepine as needed the week before remission than those whose illness had not remitted.
Discussion
Results from this prospective longitudinal study of anxiety disorders indicate that despite attempts aimed at increasing the use of SSRIs to treat panic disorder (e.g., APA practice guideline), there has only been a modest increase in the proportion of patients receiving such treatment. Psychotropic treatment patterns appear to have remained relatively stable over the past decade (1989–2001), with benzodiazepines being the most common class of drugs used to treat panic disorder. In comparison, the use of SSRIs throughout the follow-up period has remained low. A majority of the patients taking an SSRI also reported taking a benzodiazepine concomitantly. A closer inspection of the patients who were experiencing an episode of panic disorder at each of the follow-up intervals revealed similar psychotropic treatment patterns over time. These results are consistent with those of Uhlenhuth and colleagues
(16), who reported a negligible decrease in the overall frequency of expert recommendations for benzodiazepines as a first-line treatment for panic disorder over a 5-year interval in the 1990s.
The effect of comorbid major depressive disorder on medication treatment patterns in patients with panic disorder was found to be a significant predictor of greater use of SSRIs. This is consistent with an earlier study that examined pharmacological treatment of generalized anxiety disorder
(17), in which we also found significant increases in the use of SSRIs when accompanied by comorbid depression. However, the presence of other comorbid conditions, such as alcohol or substance use, did not increase SSRI use. This was unexpected since we had initially hypothesized that use of SSRIs would be greater in these patients because of concerns associated with the combination of alcohol and benzodiazepines.
Overall, we were somewhat surprised by these findings. With the advent of several new SSRIs during the 1990s, we had anticipated a marked increase in the use of SSRIs over time, particularly during the most recent follow-up intervals. Why would a high percentage of these patients’ physicians not prescribe SSRIs for panic disorder, despite treatment guidelines endorsing SSRIs as “the most favorable balance of efficacy and adverse effects” in many patients with panic disorder
(8)? Potential connections for the observed low levels of use of SSRIs have been posited by researchers, and collectively, they may help explain the observed levels of SSRI use in patients with panic disorder. For example, Lomas and colleagues
(18) examined the influence of practice guidelines on a group of physicians. Their findings indicated that although physicians’ self-reports indicated a change in the way in which they practice, actual behavior had changed little. Other studies have found resistance to practice guidelines, particularly with more experienced physicians
(19,
20). Resistance appears to be due, in part, to fear of loss of autonomy and clinical judgment, as well as to problems with the practical application of such guidelines.
Dugan and Cohen
(21) suggested that “before physicians view guidelines as precise tools to use in the construction of optimal patient care, they must be convinced that guidelines contain clear, evidence-based recommendations, and that there is a distinct advantage to them in changing practice styles to follow guidelines” (pp. 284–285). For some physicians and patients, the rapid onset of action of the benzodiazepines make their use more favorable than the newer SSRIs, which can take weeks to provide any benefit. This may be shown by the high rate of patients using benzodiazepines on an as-needed basis. Additionally, the comparatively high rates of noncompliance with the taking of SSRIs found in some studies because of unwanted sexual side effects may make the “distinct advantage” of using an SSRI murkier. For example, discontinuation of SSRIs in patients with panic disorder has been attributed to adverse side effects 52% of the time, in contrast to benzodiazepines, for which adverse side effects were responsible for only 7% of the discontinuation
(22). In a recent study of 6,299 primary care outpatients, 36%–43% of the patients taking an SSRI reported sexual dysfunction
(23), which is much higher than the rate of sexual dysfunction reported in the product literature. A physician who has had multiple patients stop using SSRIs because of sexual dysfunction or other side effects may find these treatment guidelines overly theoretical and too divorced from their patients’ concerns regarding quality of life.
Two important observations must be noted. First, since this study did not include data from patients’ treating physicians, it is impossible to determine whether SSRIs were not being prescribed to the patients or whether the physicians prescribed SSRIs, but the patients failed to take them because of concerns regarding potential unwanted side effects. Lack of patient education surrounding these possible side effects and education regarding the 4–6-week time period for the medication to become effective may also play a part in the low rates of SSRI use. Second, the patients recruited for this study had a long history of panic disorder and originally sought treatment more than a decade ago. Since the patients were recruited from treatment settings from 1989 to 1991, most SSRIs had not yet become available, and thus, many were initially treated with a benzodiazepine. Therefore, the findings from this study may not be indicative of individuals who are newly diagnosed with panic disorder and seeking treatment for the first time.
These results highlight an existing gap between recommended pharmacological treatment guidelines and actual delivery of care for the treatment of panic disorder. It appears that the recommendation to use SSRIs as a first choice is not being followed rigorously. Future studies should address this apparent gap, including whether or not the clinical guideline to use SSRIs as a first-line treatment is premature. In an effect-size analysis of SSRIs for the treatment of panic disorder, Otto and colleagues
(24) found no evidence to support the hypothesis that SSRIs are more effective than older antidepressants for the treatment of panic disorder. They suggest that earlier studies with small sample sizes may have led to an initial overestimation of the efficacy of SSRIs for panic disorder. To our knowledge, no large controlled comparison study has been conducted to examine the superiority of SSRIs over benzodiazepines for the treatment of panic disorder. Thus, it may be difficult for some physicians to alter prescribing patterns until such firm evidence exists for the superiority of SSRIs.
Although our results indicate that the patients whose panic disorder remitted were no more likely to be taking SSRIs or benzodiazepines (or both in combination) before remission, the lack of treatment effects on their illness’ clinical course was not unexpected. Since the Harvard/Brown Anxiety Research Project is an observational study of anxiety disorders by design, it observes but does not manipulate the treatment received by its patients. Treatment analyses performed by the Harvard/Brown Anxiety Research Project to date have shown that the treatment the patients received does not play a decisive role in improving the course of their anxiety disorders, since we have shown relatively slow rates of recovery and/or high rates of recurrence
(11). This could be because of our findings of low doses of medication for the treatment of anxiety disorders and minimum treatment with empirically based psychotherapies with demonstrated efficacy or because of self-selective biases that make treatment appear to have negative effects because there is no experimental control over treatment.
It is important to note that a substantial number of patients in this study were not taking a benzodiazepine or an SSRI. A few patients were receiving older medications, such as MAOIs (2%), imipramine (9%), and buspirone (5%), at intake. Additionally, other newer medications, such as venlafaxine, also had low rates of use during follow-up (5% at the 10-year follow-up). Recruitment occurred in specialized treatment settings, so individuals receiving somatic treatment for their panic disorder is likely to be lower in community settings. These results, when combined with those from earlier studies reporting the low rates of patients from the Harvard/Brown Anxiety Research Project who were receiving empirically supported psychosocial treatment for their panic disorder (e.g., cognitive behavior therapy)
(25,
26), indicate that a substantial number of patients in our group were not receiving any of the recommended treatment for their panic disorder symptoms. Although the continuation of controlled clinical trials is paramount, these findings also highlight the need for increased effectiveness research to examine the factors associated with promoting and inhibiting the implementation of treatment guidelines for panic disorder in real-world settings. These factors may help improve and modify existing treatment guidelines and thus help minimize the gap between clinical research and actual delivery of care.
Acknowledgments
The Harvard/Brown Anxiety Disorder Research Program is conducted with the participation of the following investigators: M.B. Keller, M.D., Chair: M.T. Shea, Ph.D. (Veterans Administration Hospital, Providence, RI; Brown Medical School); J. Eisen, M.D., K. Phillips, M.D., R. Stout, Ph.D. (Butler Hospital, Brown Medical School); S.E. Bruce, Ph.D., R.B. Weisberg, Ph.D., M.G. Warshaw, M.S.S., M.A. (Brown Medical School); R.M. Goisman, M.D. (Massachusetts Mental Health Center, Harvard Medical School); A. Massion, M.D. (University of Massachusetts Medical Center); M.P. Rogers, M.D. (Brigham and Women’s Hospital, Harvard Medical School); C. Salzman, M.D. (Massachusetts Mental Health Center, Harvard Medical School); G. Steketee, Ph.D. (Boston University School of Social Work); K. Yonkers, M.D. (Yale University School of Medicine); I. Goldenberg, Psy.D.; G. Mallya, M.D. (McLean Hospital, Harvard Medical School); T. Mueller, M.D. (Butler Hospital, Brown Medical School); F. Rodriguez-Villa, M.D. (McLean Hospital, Harvard Medical School); R. Vasile, M.D. (Beth Israel Deaconess Medical Center, Harvard Medical School); C. Zlotnick, Ph.D. (Butler Hospital, Brown Medical School); E. Fierman, M.D. Additional contributions were from the following: P. Alexander, M.D. (Butler Hospital, Brown Medical School); J. Curran, M.D.; J. Cole, M.D. (McLean Hospital, Harvard Medical School); J. Ellison, M.D., M.P.H. (Harvard Pilgrim Health Care, Harvard Medical School); A. Gordon, M.D., S. Rasmussen, M.D. (Butler Hospital, Brown Medical School); R. Hirschfeld Ph.D. (University of Texas, Galveston); J. Hooley, D.Phil. (Harvard University); P. Lavori, Ph.D. (Stanford University); J. Perry, M.D. (Jewish General Hospital, McGill University School of Medicine, Montreal); L. Peterson (Midcoast Medical Group, Rockport, Maine); J. Reich, M.D., M.P.H.; J. Rice, Ph.D. (Renard Hospital, Washington University School of Medicine); H. Samuelson, M.A. (Brigham and Women’s Hospital); D. Shera, M.S. (Harvard School of Public Health); N. Weinshenker, M.D. (New Jersey Medical School); M. Weissman, Ph.D. (Columbia University); K. White, M.D.