Autistic disorder (autism) is characterized by a significant impairment in social interaction and communication and a markedly restricted repertoire of activity and interests. Although the etiology is unknown, immune system dysfunction has been hypothesized to contribute to the pathophysiology
(1).
Genetic mechanisms play an important part in immune system regulation, as well as autism. Immunomodulatory genes in the human leukocyte antigen (HLA) region of chromosome 6 have been implicated in autism in some studies. These genes include certain HLA extended haplotypes, the third hypervariable region of DRβ1, the complement C4B null allele, and HLA-DR4 and HLA-DR13
(2,
3). Furthermore, evidence of immune system activation, including an abnormal CD4:CD8 T cell ratio
(1), a high number of DR+ (activated) T cells
(4), high urinary neopterin levels
(5,
6), and high cytokine production
(7,
8), has been reported.
Most prior investigations of immune cell counts in autism have focused on monocyte-depleted mononuclear cell samples
(9). Monocytes circulate in the blood and continually differentiate into macrophages upon migration into the surrounding tissues. Macrophages, in turn, phagocytize pathogens and present antigens to lymphocytes. When stimulated by the cytokine interferon gamma, monocytes and macrophages produce increased amounts of the molecule neopterin. High levels of neopterin serve as an indicator of monocyte/macrophage activation, as well as activation of T cells and cell-mediated immunity
(10).
We are aware of one prior study of monocyte counts in autistic children
(9). Mean blood absolute monocyte counts and percentages were reported for 10 children with autism and 10 matched healthy comparison subjects. The autistic subjects had a somewhat higher absolute monocyte count and a significantly higher percentage of monocytes, in relation to total leukocytes, than the comparison subjects. Previous measurements of neopterin have indicated higher urinary levels in autistic subjects than in comparison subjects
(5,
6). However, when neopterin was measured in combination with its precursor dihydroneopterin, urine levels were similar
(5) and plasma levels were lower
(11) in autistic subjects than in comparison subjects. Measurements in cerebrospinal fluid have shown similar neopterin levels in autistic and comparison subjects but lower dihydroneopterin levels in autistic subjects
(12).
Enzyme-linked immunosorbent assay (ELISA) is a novel method for neopterin detection. In the present study we used this method, as well as standard immune cell counts, to assess immune system activity in children with autism. To improve on previous work, plasma neopterin levels were measured in a larger group of medication-free, well-characterized children with autism and in age-, race-, and gender-matched healthy comparison subjects.
Method
The study was approved by the Indiana University institutional review board. Written informed consent was obtained from each subject who was able to provide written consent and each subject’s parent or legal guardian after a complete description of the study. We recruited children with autism from the Christian Sarkine Autism Treatment Center of the James Whitcomb Riley Hospital for Children. Of the 31 total, four were girls and 27 were boys; their mean age was 6.0 years (SD=2.8, range=2–12). All subjects met the DSM-IV criteria for a clinical diagnosis of autistic disorder as determined by a board-certified child and adolescent psychiatrist (D.J.P. or C.J.M.). The Autism Diagnostic Interview—Revised
(13) was administered to confirm the diagnosis. Intelligence was measured by using the Leiter International Performance Scale—Revised
(14) or the Wechsler Intelligence Scale for Children, 3rd edition
(15), for the 23 autistic children who were able to comply with testing.
Age-, race-, and gender-matched healthy comparison children were recruited from the surrounding community by means of newsletters and flyers. Of the 28 total, four were girls and 24 were boys; their mean age was 6.5 years (SD=2.5, range=2–10). Each of the children with autism and comparison subjects was medication free for at least 5 weeks and was given a physical examination to screen for evidence of immune activation, such as elevated temperature, infection, inflammation, or malignancy, before blood was drawn.
Blood was drawn into EDTA tubes between 7:00 a.m. and 10:00 a.m. after overnight and morning fasting. All cell counts were screened by one of us (D.J.P. or C.J.M.) for indicators of infection or other abnormalities. Cell counts were not available for one autistic child and one comparison subject. Numbers of neutrophils, eosinophils, basophils, lymphocytes, monocytes, and total leukocytes were determined by flow cytometry in a blinded fashion at the Indiana University Hospital reference laboratories. For measurement of neopterin levels, blood was protected from light, centrifuged to obtain plasma, and frozen at –40°C before blinded assay in duplicate by ELISA. This assay has an intra-assay coefficient of variance of 5.5% and an interassay coefficient of variance of 10.3% at a concentration of 7 nmol/liter.
Two-tailed group t tests and correlation analysis were performed by using SPSS statistical software (Chicago, SPSS).
Results
No abnormalities indicative of immune activation were identified during physical examination or in the screening blood work for any of the children with autism or comparison subjects. However, the group mean absolute monocyte count was significantly higher in the autistic children (mean=0.588×103 cells/mm3, SD=0.215) than in the normal comparison subjects (mean=0.491×103 cells/mm3, SD=0.143) (t=2.01, df=50.8, p<0.05). No differences between groups were found in the total absolute leukocyte count, in the neutrophil, eosinophil, basophil, or lymphocyte count or percentage, or in the percentage of monocytes.
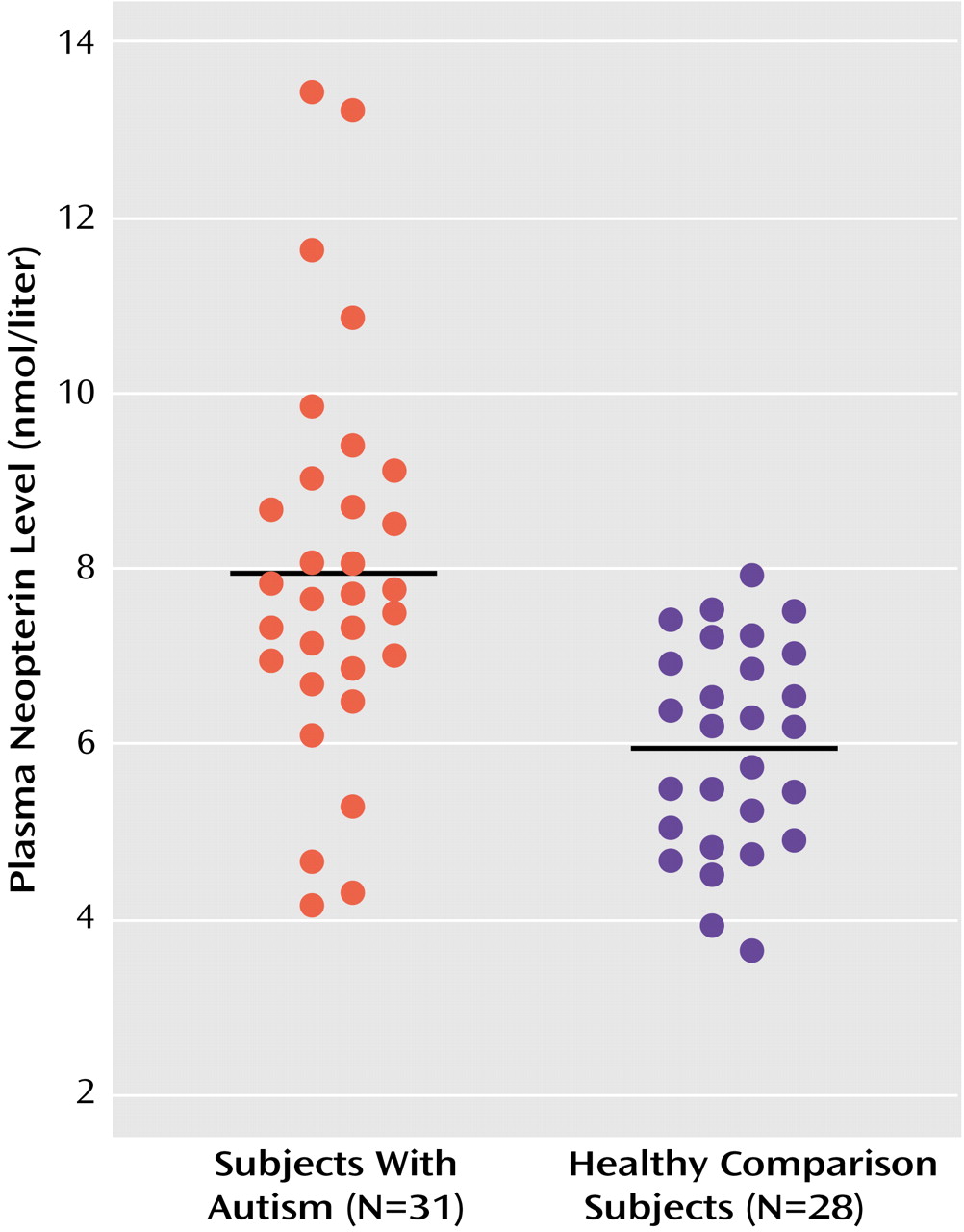
Plasma neopterin levels were also significantly higher in the autistic group (mean=7.94 nmol/liter, SD=2.21) than in the comparison subjects (mean=5.95 nmol/liter, SD=1.18). All but four of the autistic subjects had neopterin levels greater than the mean value of the comparison subjects, and no comparison subject had a neopterin value higher than the mean value of the autistic subjects (
Figure 1). There was no correlation between neopterin level and age or monocyte count in either of the groups. In the autistic children with available IQs (mean IQ=79, SD=23, range=36–109), there was no correlation between IQ and monocyte count or neopterin level.
Discussion
High blood monocyte counts are generally found in autoimmune and some infectious disease states. High neopterin levels are a well-established marker of immune activation, consistent with heightened cell-mediated immunity
(10). High plasma neopterin levels are found in various immune-related clinical disease conditions
(10). The higher plasma monocyte counts and neopterin levels reported here in autism are similar in magnitude to those seen in multiple sclerosis
(16). Serum neopterin levels have also been found to be high in neuropsychiatric disorders in which inflammatory processes may be active, such as Alzheimer’s disease
(17).
Although no correlation was found between monocyte count and neopterin level, their concurrent elevation indicates monocyte/macrophage activation, with heightened cell-mediated immunity in the autistic subjects. This pattern of response is consistent with two recent findings of high in vitro production of cytokines involved in cell-mediated immunity and monocyte/macrophage activation in immune cells from autistic children
(7,
8). Additional research into immune system function in autism appears warranted.