Recent neuroimaging studies have supported the notion of frontal cortical dysfunction in ADHD and have specifically implicated dysfunction within frontal-striatal circuits as a putative mechanism associated with impulsive tendencies of ADHD children. Structural magnetic resonance imaging (MRI) studies have found abnormal volumes in both the frontal lobes
(9–
11) and basal ganglia
(10,
12,
13). In addition, functional imaging studies relying on positron emission tomography and single photon emission computed tomography have demonstrated reduced metabolism in frontal and striatal regions in ADHD
(14–
16). Most recently, functional MRI (fMRI) studies have demonstrated frontal lobe and striatal dysfunction in ADHD during the performance of inhibitory tasks, including the go/no-go and stop tasks
(17–
19) and the counting Stroop test
(20).
While these studies lead to a converging view regarding the neurobiological foundations of impulsivity in ADHD, most have neglected to address the attentional component of the disorder. It is essential to establish the neural circuitry responsible for attentional deficits in ADHD because inattention is a core feature of the disorder
(21). Furthermore, the only fMRI study of the effects of stimulant medication on ADHD
(18) did not directly address the medication’s effect on the neural circuitry of attention. Therefore, the current study was designed to investigate three unresolved questions regarding the neurobiological foundations of ADHD. First, what are the neural systems responsible for the attentional deficit in ADHD? Second, does the stimulant methylphenidate modulate the activity of these regions? Finally, are the neural deficits and effects of methylphenidate specific to ADHD, or can similar effects be seen in related developmental disorders, such as reading disorder?
Method
Subjects
The subjects were 15 adolescents (11 boys and four girls, ages 14–17) who were diagnosed with ADHD, combined type; eight adolescents (six boys and two girls, ages 12–17) who were diagnosed with reading disorder; four adolescents (all boys, ages 14–18) with a primary diagnosis of reading disorder who also met DSM-IV criteria for ADHD; and 14 healthy comparison subjects (seven boys and seven girls, ages 12–20).
Subjects in the ADHD and reading disorder groups were recruited from a large cohort of adolescents who participated in previous attention and reading studies at the Yale Center for the Study of Learning and Attention. DSM-IV diagnoses of ADHD were determined from structured clinical interviews (the Diagnostic Interview Schedule for Children, Version 2.3)
(23). Exclusion criteria included the presence of comorbid disorders other than oppositional defiant disorder or conduct disorder. The criteria for reading disorder were met if the average of the word identification and the word attack subtests of the Woodcock-Johnson Psychoeducational Test Battery
(24) were below a standard score of 90 (below the 25th percentile) or 1.5 standard error of the mean below the expected reading achievement score when we used the WISC-III
(25) full-scale IQ
(26). Of the 15 subjects with ADHD only, eight were currently being treated with methylphenidate, and an additional four reported a history of methylphenidate therapy. In addition, four subjects with reading disorder only and three subjects with reading disorder plus ADHD reported previous drug therapy with methylphenidate.
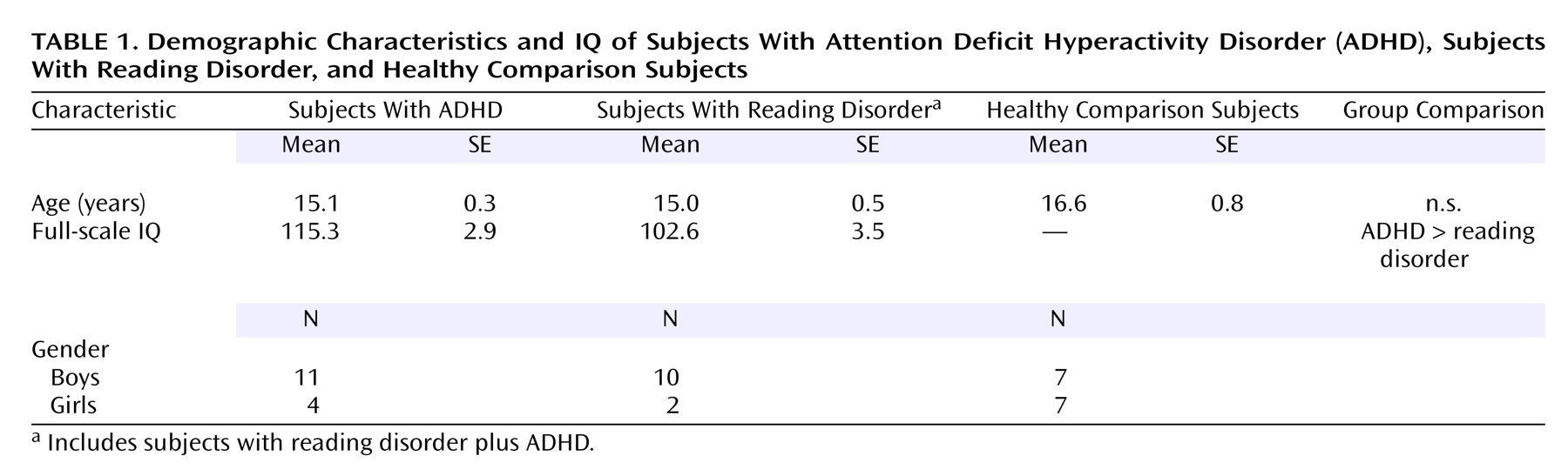
Table 1 summarizes the subjects’ demographic and IQ data.
Subjects in the healthy comparison group were recruited from the local area and were free of any history of learning disabilities or psychiatric or neurological problems, as determined through detailed interviews with both the participants and their parents. This study was approved by the Yale School of Medicine Human Investigations Committee, and all subjects gave informed written consent for their participation.
Testing Procedure
Each subject in the ADHD and reading disorder groups was tested during two sessions—once when given methylphenidate and once when given placebo. The study was conducted in a double-blind crossover fashion, with testing sessions approximately 1 week apart and the order of sessions counterbalanced across subjects. Approximately 1.25 hours before imaging, the subjects were given methylphenidate hydrochloride or placebo (lactose), with the dose for each subject adjusted for weight with the following guidelines: under 30 kg=15 mg, 30 to 60 kg=20 mg, more than 60 kg=25 mg.
Because of the ethical considerations of giving methylphenidate to healthy individuals, subjects in the comparison group did not participate in the medication trial.
fMRI Tasks
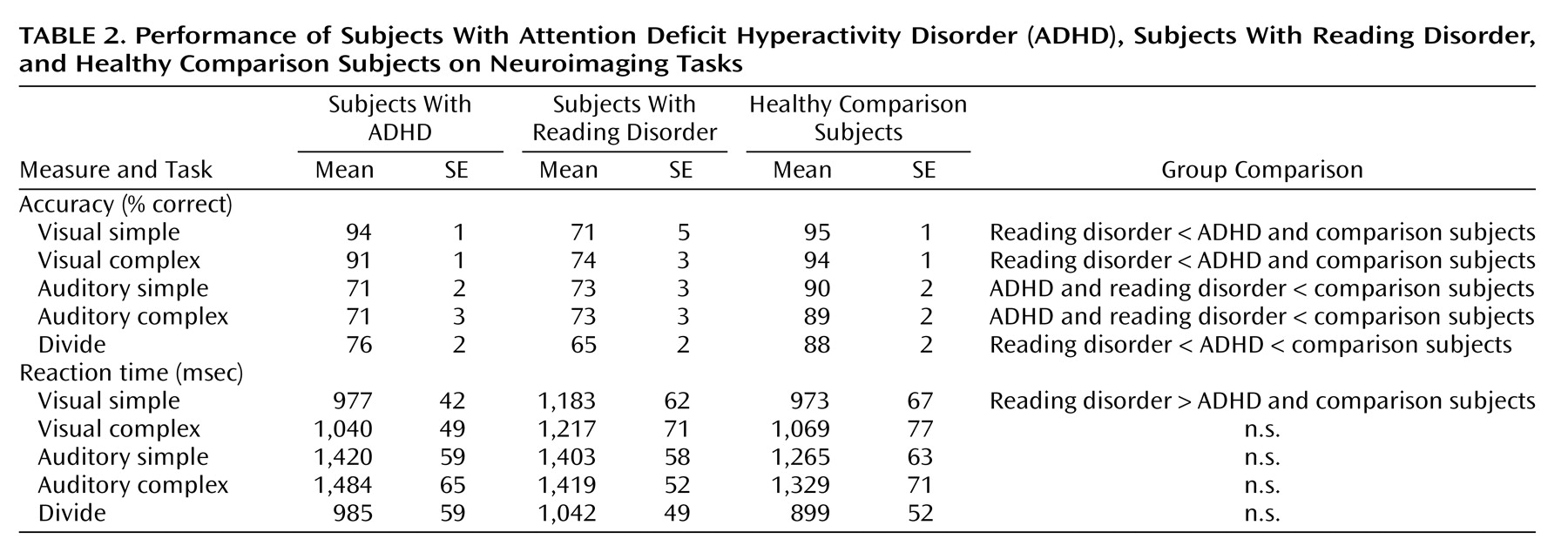
The subjects performed two attentional tasks in the fMRI—a selective attention task and a divided attention task. The selective attention task involved four experimental conditions: visual simple, visual complex, auditory simple, and auditory complex; the divided attention task consisted of one condition. An additional task involving simple button-press responses served as a baseline measure. The task conditions were presented in a block design, with four trials (4.5 seconds each) per block and 13 blocks per fMRI run.
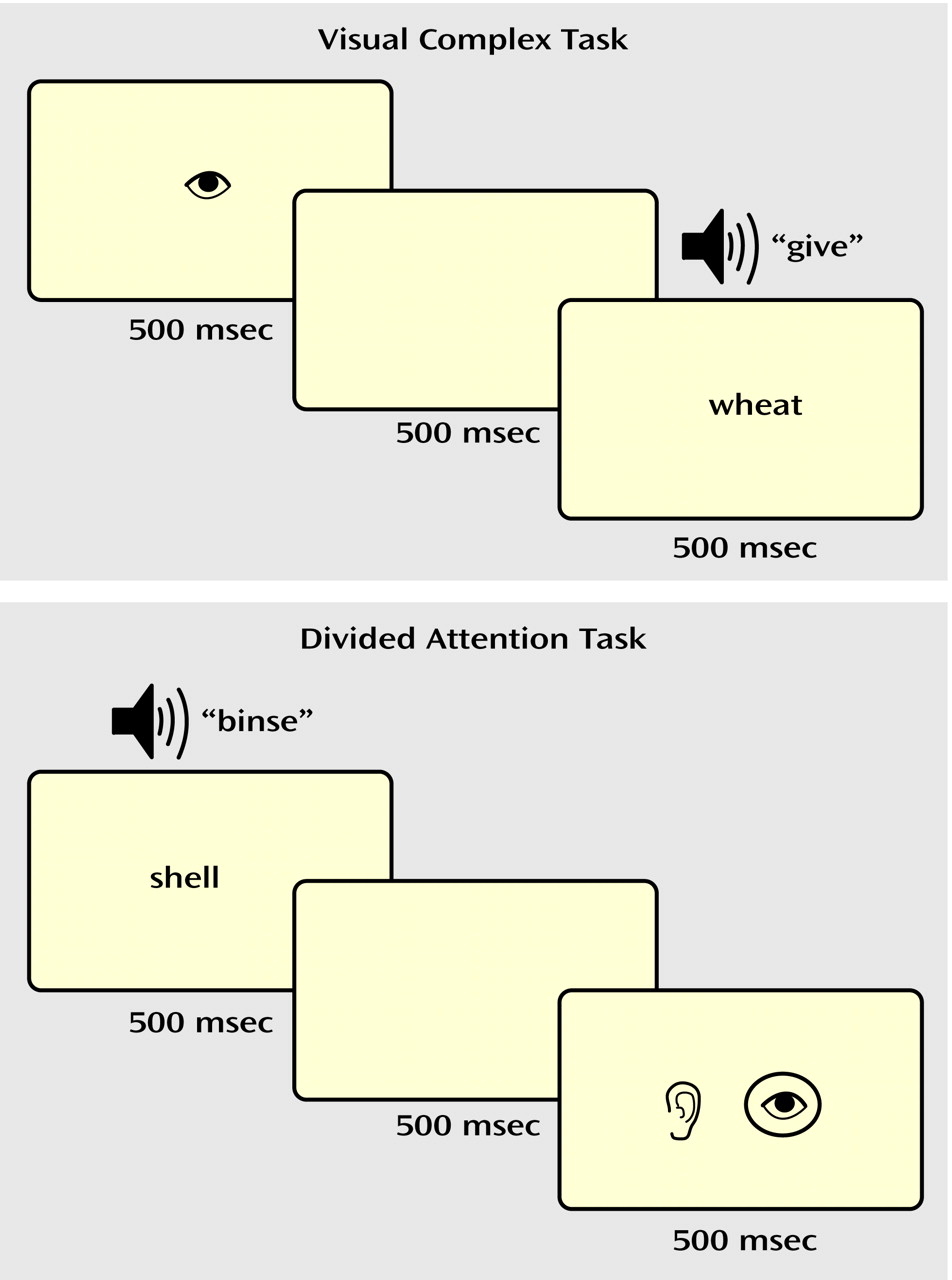
In the selective attention task (
Figure 1), a drawing of an eye or an ear (shown for 500 msec) cued the participant to attend to either the visual or auditory stimulus. After a 500-msec pause, a “target” word or pseudoword was presented in the cued modality, i.e., either projected on a screen (visual) or played through headphones (auditory). The subjects made a word/nonword judgment (i.e., yes/no lexical decision) to the target and responded with an appropriate button press. In the visual simple and auditory simple conditions, a nonlinguistic stimulus (a line array or a tone stimulus) was simultaneously presented in the unattended modality. In the visual complex and auditory complex conditions, a word or nonword distracter was simultaneously presented in the unattended modality. Thus, in the complex selective attention conditions (visual complex, auditory complex), the subjects were required to ignore a potentially confusing stimulus in the unattended modality.
In the divided attention task (
Figure 1), visual and auditory linguistic stimuli (words or nonwords) were presented simultaneously for 500 msec. After a 500-msec pause, a drawing of both an eye and an ear appeared on the screen for 500 msec, with the eye representing the visual modality and the ear representing the auditory modality. A circle appeared around both, one, or neither of the pictures. The circles were described as a “prediction” the computer gave as to whether the preceding stimulus in each modality was a real word, with the presence of a circle representing a real word. The participants determined whether the computer’s predictions were correct and responded with appropriate button presses.
The baseline control task consisted of button presses with no lexical decision. For selective attention runs, the subjects were shown a picture of a hand with a finger pointing to the right or left for 500 msec. After a 500-msec pause, a line array and a tone were presented simultaneously for 500 msec. The participants were instructed to press the button corresponding to the direction of the finger pointing. For divided attention runs, the line array and tone were presented first, followed by the hand, to better represent the stimulus presentation order for that task.
To minimize the effect of practice from one session to another, two versions of the tasks were created, each containing different sets of stimuli. The subjects were presented with one version during their first session and the other during the second session, with the order counterbalanced across subjects, independent of the random assignment of drug order.
fMRI Data Collection
fMRI scans were acquired with a 1.5-T GE LX MRI scanner (General Electric, Milwaukee) equipped with gradients for echo planar (blood-oxygen-level-dependent [BOLD]) imaging. T1-weighted anatomic images were collected in the sagittal plane by using conventional parameters, followed by 14 axial-oblique slices parallel to the anterior-posterior commissural (AC-PC) line. Functional images were acquired during eight scanning runs: six selective attention runs and two divided attention runs. The additional selective attention runs ensured that an equivalent amount of data was collected for all conditions of the tasks. Stimuli were presented by using PsyScope software and back-projected from a liquid crystal display panel onto a screen viewed by the subject through a prism mirror. The subjects responded by using a magnet-compatible button box. Echo planar imaging parameters were the following: fourteen 7-mm-thick slices parallel to the AC-PC line, TR=1500 msec, TE=60 msec, flip angle=60°, in-plane resolution=3.12×3.12 mm, acquisition matrix=64×64 pixels over a field of view of 20×20 cm.
fMRI Data Analysis
Before statistical analysis, the images from each run were motion-corrected by using the SPM 99 program. Images were discarded if the motion exceeded 2 mm of displacement or 3° of rotation in any direction. In addition, the first two images of each block were discarded to account for the delay in the hemodynamic response. The remaining images were thresholded (with the signal outside the brain set to zero) and Gaussian-filtered (full width at half maximum=6.3 mm). Statistical parametric maps of BOLD activation for each subject were created by using a skew-corrected percent signal difference for each attention task relative to its control task.
Anatomical images and activation maps from individual subjects were transformed into standardized Talairach space
(27), and the resulting maps from all subjects in each diagnostic category were superimposed to create cluster-filtered (10 contiguous pixels) composite activation maps for each of the five attention tasks. The probability that the mean percent signal change across subjects was significantly different from zero was calculated by using a t test at each composite pixel. Contrast maps were then created to examine the activation differences across medication status (within subjects) and across diagnostic categories (between subjects). Drug contrasts were created by direct statistical comparison of each subject’s activation when given methylphenidate to activation when given placebo. Group contrasts were made by comparing activation of the ADHD subjects to that of the subjects with reading disorder and of the ADHD and reading disorder subjects relative to the comparison subjects. To account for subjects with combined diagnoses of reading disorder and ADHD, two separate analyses were performed: one in which reading disorder plus ADHD subjects were included in the reading disorder group and another in which these subjects were included in the ADHD group. No differences between the two analyses were observed.
Discussion
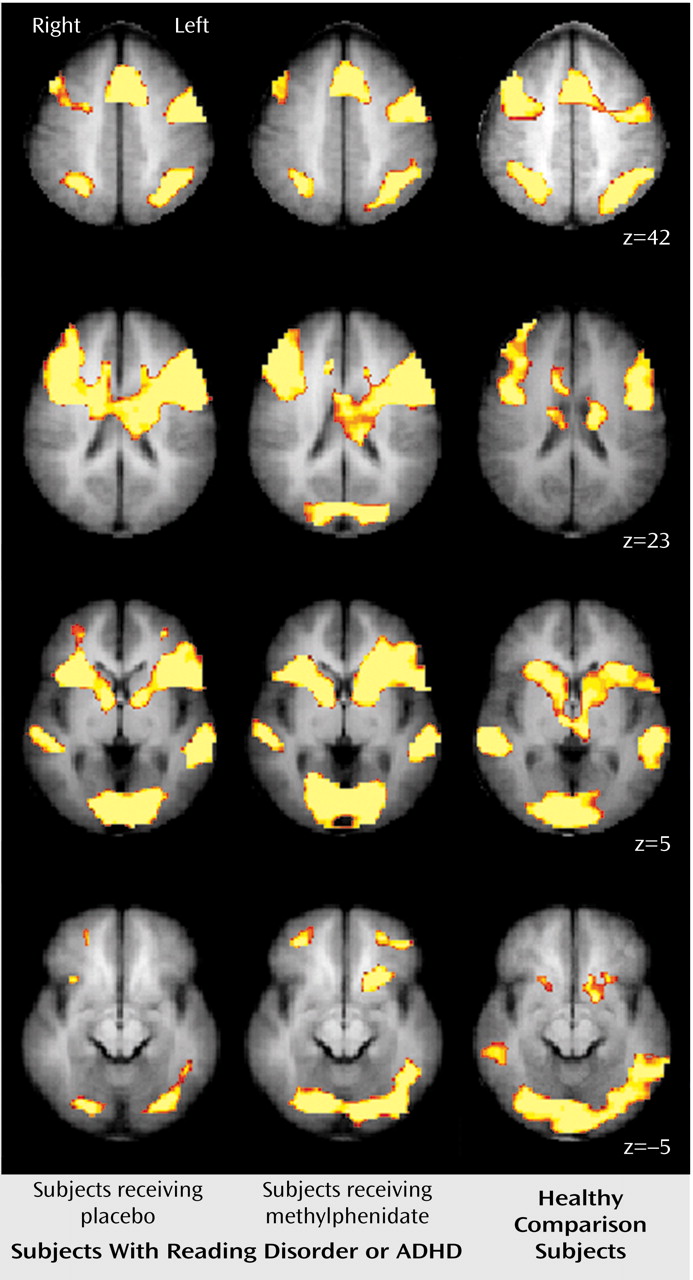
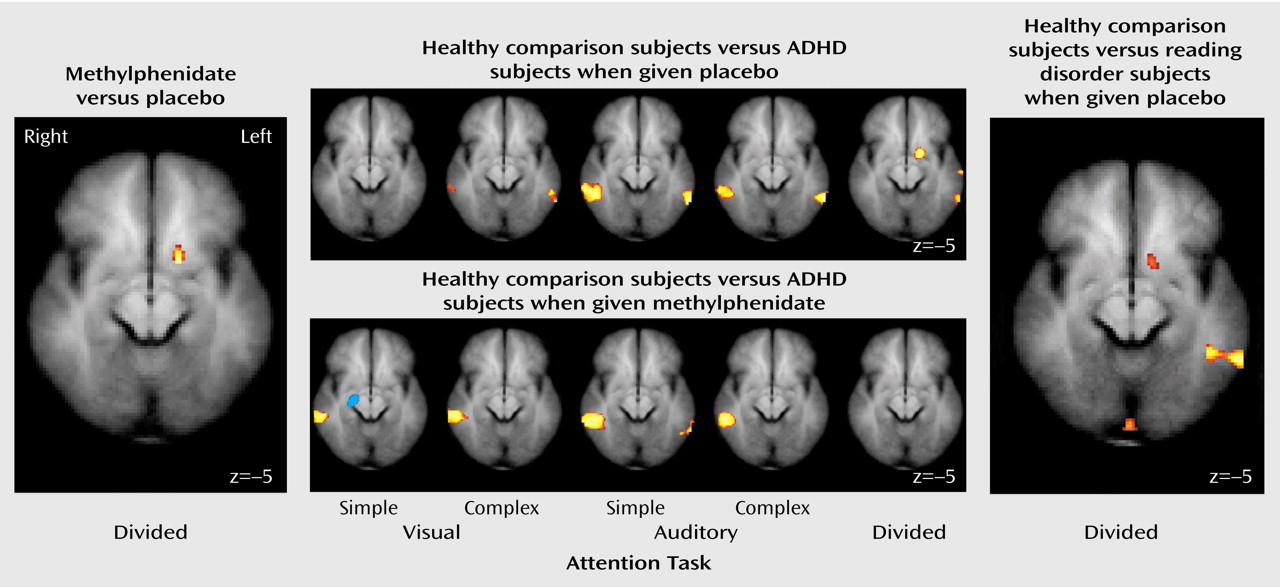
These results indicate that unmedicated ADHD adolescents differed from healthy comparison subjects in the activation of the left ventral aspect of the basal ganglia during the performance of a divided attention task. Specifically, the comparison subjects activated this region to a greater extent than the ADHD participants when given placebo. When the ADHD adolescents were given a challenge dose of methylphenidate before scanning, they recruited this region of the basal ganglia to a similar degree as the comparison subjects. Similarly, unmedicated reading disorder adolescents showed a reduction in activation of the left striatum relative to healthy comparison subjects during the divided attention task, and a challenge dose of methylphenidate normalized activation in this region. Although performance on the task differed between the ADHD, reading disorder, and comparison adolescents, neither measure of performance was correlated with activation in the striatum. Therefore, these activation differences cannot be accounted for simply by performance differences. Additionally, the comparison subjects recruited the posterior aspect of the middle temporal gyrus to a greater extent than the ADHD adolescents during the attention tasks. These differences also appear to be independent of any consistent pattern in task performance. The lack of a correlation between IQ and striatal activation suggests that any IQ differences between the ADHD and reading disorder adolescents did not significantly affect our neuroimaging findings.
Our results indicate that adolescents with either ADHD or reading disorder show quite similar neural activation patterns to healthy comparison subjects during the performance of selective and divided attention tasks. For all subjects, the selective and divided attention tasks recruited a host of cortical areas, including the dorsolateral prefrontal cortex, the anterior cingulate cortex, the premotor cortex, and Broca’s area and, particularly for divided attention, the posterior parietal area and regions of the basal ganglia. These findings serve to reinforce the role of these cortical and subcortical structures in the processing of attention-related information and the generation of behavioral responses and indicate that adolescents with either ADHD or reading disorder successfully recruit the majority of these regions.
Our findings of reduced striatal activation for adolescents with ADHD are consistent with prior neuroimaging studies showing that ADHD subjects exhibit less activity in basal ganglia structures both at rest and during the performance of cognitive tasks
(14,
15,
18,
19,
28). Moreover, our finding that methylphenidate normalized striatal activation is consistent with previous reports that methylphenidate preferentially modulates striatal activity in ADHD subjects
(14,
15,
18) and increases extracellular dopamine in the striatum in healthy adults
(29). While we did not observe differences in frontal cortical functioning, this finding is supported by prior fMRI results showing that frontal lobe activity may be more related to task parameters than to either diagnostic or medication status
(18). A lack of consistent neuropsychological findings in children and adolescents with ADHD
(30) supports the notion that frontal cortical deficits in ADHD may be subtle and related only to specific components of executive functioning.
An intriguing finding in the current study is that methylphenidate increased striatal activation for both the ADHD and reading disorder adolescents. These results suggest that methylphenidate may have similar modulatory effects in certain brain regions, whether or not an attention disorder is present. Our findings fit well with earlier research demonstrating that low-dose psychostimulants can enhance cognitive performance and reduce impulsiveness in individuals other than those with ADHD, including normal children and adults
(31,
32).
In our study, methylphenidate significantly increased striatal activity only during the divided attention task. This task specificity is not surprising after accounting for the neural structures engaged by the various task conditions. Relative to the baseline sensorimotor task, the basal ganglia were preferentially activated only by the divided attention task. One interpretation of this pattern is that the divided attention condition required additional cognitive processing over the selective attention conditions. More specifically, the selective attention conditions required a two-stage cognitive process in which the participant first encoded the auditory and visual stimuli and then made a judgment as to whether the stimulus in the attended modality was a real word or a pseudoword. In the divided attention task, an additional step required participants to determine the accuracy of a prediction by the computer as to the lexical nature of the previously presented stimuli.
It is reasonable to suggest that the selective attention conditions, when directly compared to the baseline task, did not strongly engage the basal ganglia because these tasks were not demanding enough to require additional processing afforded by basal ganglia–cortical circuitry. Because the divided attention task was designed to strongly engage executive processes, that task led to increased activation of the basal ganglia relative to the sensorimotor task. Therefore, we suggest that methylphenidate modulated striatal activity only during the divided attention task because it was during this task that these structures were preferentially activated.
The current findings provide novel information about the regional neural effects of methylphenidate during attentional control. However, the mechanisms by which the actions of methylphenidate translate into the improvement often seen in the cognitive and behavioral symptoms associated with ADHD remain unknown. The enhanced activation of the striatum by methylphenidate may reflect an increase in neural processing related to the inhibition of prepotent or impulsive responses
(18) or the selection and execution of appropriate behavioral responses
(33). Alternatively, methylphenidate-induced increases in dopamine activity within the striatum may increase the motivational salience
(29,
34) of the task without necessarily making the task easier to perform. Our results are consistent with both interpretations.
The role of the reduced activation in temporal lobe regions observed in ADHD adolescents is more difficult to determine. There is little prior evidence suggesting that ADHD is associated with abnormalities in temporal cortical circuitry. However, our findings of reduced activation of the middle temporal gyrus in ADHD participants were quite robust, particularly for the auditory selective tasks. The region of the middle temporal gyrus in which these differences occurred lies in the same area of the temporal lobe associated with neural deficits in dyslexia
(26). We suggest that this region of the middle temporal gyrus mediates attentional processing for verbal stimuli and may be particularly important when attending to auditory information. Further investigation into the role of the middle temporal gyrus in attention and ADHD is warranted.
In summary, our findings demonstrate neural dysfunction and neural effects of methylphenidate in adolescents with ADHD or reading disorder during the performance of attention tasks. While our ADHD group was larger than in previous fMRI studies of ADHD, the current study was potentially limited by the small size of the group with reading disorder only. The size of our reading disorder group, though, is consistent with many reported fMRI studies, particularly those involving clinical populations. Moreover, our imaging results demonstrating striatal dysfunction for both ADHD and reading disorder participants indicate that we had sufficient power to draw meaningful conclusions from the reading disorder group. Nevertheless, replication of this study with a larger reading disorder group would reveal with more certainty whether neural deficiencies underlying poor attentional control are specific to ADHD or are observed as well in other developmental disorders.
We also note that most of the ADHD subjects and a number of the reading disorder subjects had a history of drug therapy with methylphenidate before this study. While we attempted to control for medication history by ensuring that participants were medication free for at least 72 hours before testing, this does not eliminate possible long-term modulation of neural functioning stemming from methylphenidate use. Thus, it is unclear whether any additional neural effects of methylphenidate would be seen for ADHD subjects with no prior history of medication use. Despite these limitations, our findings are consistent with current conceptions of ADHD and provide novel insights into the neural mechanisms underlying the effect of methylphenidate on attentional control.