Cigarette smoking is recognized as an addiction. Long-term abstinence rates are poor, averaging between 20% and 35% for the most intensive and widely accepted treatments
(1–
4). For most addictive drugs, the recognition that addiction is chronic and relapsing has led to treatment of extended duration, follow-up support, and, when feasible, easy reentry into treatment. For cigarette smoking, however, promulgated models have been inexpensive and time limited; these include large-scale community intervention trials (for example, COMMIT
[5]) or brief courses of medications or limited psychological contact
(1,
6).
The few extended treatments tested for tobacco dependence have met with some success. Hays et al.
(7) treated subjects for 7 weeks with open-label bupropion. At the end of open-label treatment, 59% of the subjects were abstinent and entered into the 52-week relapse-prevention phase. During this phase, the subjects received brief monthly individual counseling from research staff and either active or placebo bupropion. Point-prevalence abstinence was significantly higher in the bupropion group than in the placebo group at week 52 and at week 78, but the conditions did not differ at week 104. The median time to relapse was significantly greater for the bupropion group than for the placebo group.
The design was a two-by-two factorial, with treatment length (12 versus 52 weeks) and doses (active drug versus placebo) as factors. The primary hypothesis was that extended nortriptyline would be more likely to produce repeated 7-day point-prevalence abstinence at weeks 24, 36, and 52 than the remaining three treatment conditions. Secondary hypotheses were 1) that active drug would be more efficacious than placebo in producing 7-day point-prevalence abstinence across the 52-week period and 2) that extended treatment would be more efficacious than brief treatment in producing 7-day point-prevalence abstinence across the 52-week period.
Method
Subjects
Study treatments were approved by the institutional review board of the University of California, San Francisco, before recruiting was initiated. One hundred sixty subjects who smoked ≥10 cigarettes per day were stratified on baseline number of cigarettes, history of nicotine-replacement therapy versus none, and history of major depressive disorder versus none and randomly assigned to one of the four experimental cells. Subjects were recruited by advertising, public service announcements, and flyers. After telephone screening, they were invited to an orientation meeting, where they completed written informed consent and were invited to a baseline assessment including a physical examination, an ECG, and blood draws for basic blood chemistry analyses. The sections of the Structured Clinical Interview for DSM-IV (SCID)
(12) that diagnose depression, dysthymia, and bipolar disorder were administered by master’s-level clinicians.
Exclusionary criteria included cardiovascular disease, history of seizure, severe allergies, life-threatening disease, bipolar disorder, current major depressive disorder, use of l-dopa, migraine headaches, current use of any psychiatric medication including bupropion, suicidal or psychotic symptoms, current use of nicotine-replacement therapy, previous treatment for cigarette smoking with nortriptyline, treatment for drugs or alcohol within 6 months, psychiatric hospitalization within 1 year, and pregnancy or lactation.
Assessment and Measures
Assessments were repeated at weeks 12, 24, 36, and 52. Expired carbon monoxide and urinary cotinine were assessed to confirm smoking status at weeks 24, 36, and 52; carbon monoxide only was assessed at week 12. Seven-day point-prevalence abstinence at each assessment was the primary outcome measure, with subjects considered abstinent if they answered “no” to the question, “In the past 7 days, have you smoked as much as a puff of a cigarette?” and had carbon monoxide levels of ≤10 ppm and cotinine levels of ≤50 ng/ml, as currently recommended
(13). Side effects were assessed by checklist at weeks 1 through 7, 11, and 12 for all subjects and at each monthly contact after week 12 for subjects assigned to extended treatment. Based on the SCID, the subjects were classified as positive or negative for a history of major depressive disorder at baseline. We administered the Fagerstrom Test for Nicotine Dependence
(14) and the Minnesota Nicotine Withdrawal Questionnaire, a well-validated instrument that yields a total withdrawal discomfort score
(15,
16). We also administered a demographic questionnaire. Subjects were reimbursed $25.00 for returning for assessment at weeks 24, 36, and 52.
Summary of Treatment Procedure
All subjects participated in 12 weeks of brief treatment. During that time, they participated in five group counseling sessions. Subjects began active or placebo nortriptyline at week 1, and all continued taking active drug or placebo to week 12. The quit date was set at week 5 to allow therapeutic blood levels to be achieved before cessation. On the quit date, the subjects also began an 8-week course of a transdermal nicotine patch. Patch treatment continued until week 12. At week 12, the subjects were told whether or not they would be receiving extended treatment. Subjects in brief treatment had no further therapeutic contact. Subjects in extended treatment continued taking either active nortriptyline or placebo, with assignment remaining as it had been during the brief treatment period, and participated in monthly psychological counseling, with telephone counseling 2 weeks after each session except the final one. The study took place in a university-affiliated smoking-cessation clinic.
Treatment Interventions
Nicotine-replacement therapy
All subjects received 4 weeks of 21 mg of a transdermal nicotine patch, 2 weeks of a 14-mg patch, and 2 weeks of a 7-mg patch. Use of a nicotine patch began on the quit date at week 5 and continued through week 12.
Active nortriptyline versus placebo
Nortriptyline drug dose was titrated for each subject until a therapeutic serum level (50–150 ng/ml) was obtained. All subjects assigned to active nortriptyline received 25 mg/day for 3 days, followed by 50 mg/day for 11 days. The dose increased to 75 mg/day at week 3. At week 4, serum levels were assessed, and if a therapeutic serum level had not been reached, the drug dose was increased to 100 mg/day. At week 6, serum levels were assessed to determine the final dose. When the dose was titrated for a subject receiving active drug, the dose was also titrated for a randomly selected subject from the same cohort who was receiving placebo. Titration was performed by a physician who had no contact with the subjects or front-line staff. Front-line medical staff, internal medicine physicians, and licensed nurse practitioners were blind to drug assignment. Subjects met with clinicians at week 1 to start medication treatment and at weeks 2, 3, and 12 to review compliance and side effects. If a subject had relapsed, he or she was referred to a counselor.
Brief versus extended treatment
As in our previous studies with nortriptyline
(2,
4), brief treatment took place for 12 weeks. If subjects were assigned to one of the two brief treatment conditions, they were told of their assignment to brief treatment at week 12 and began a 5–7 day course of tapering from active drug or placebo.
Extended treatment took place up to week 52. At week 12, the subjects in the extended treatment conditions were told of their assignment to extended treatment. Subjects continued with the drug dose reached at the end of the titration period up to week 52. Subjects could choose to discontinue the drug before week 52. Subjects taking drug at week 52 were tapered from the drug at that time. Subjects were blind to drug assignment throughout the study and not informed of their drug assignment until after the week 52 assessment.
Psychological treatment was delivered by master’s-level psychologists and health educators. In all four treatment conditions (active brief, active extended, placebo brief, placebo extended), the subjects initially received behavioral group treatment that provided health-related information, facilitation of group discussion, and development and continuing modification of a personalized plan to quit smoking. Methods used included monitoring of cigarette use and paper-and-pencil exercises focusing on health-related information, motivation to quit, decreasing relapse-related thoughts, informational handouts, and didactic presentations. Five 90-minute sessions were held during weeks 4, 5 (two sessions), 7, and 11, for a total of 7.5 hours of counseling time. In the extended condition, the subjects received group treatment as in the brief treatment conditions. Thereafter, in both active and placebo extended treatment conditions, individual counseling sessions were held once per month. Subjects received 20–30-minute sessions every 4 weeks (weeks 16, 20, 24, 28, 32, 36, 40, 44, and 48) for a total of 3 to 4.5 hours of counseling. In these sessions, counselors continued to review and reinforce the behavior-change strategies discussed during the initial group therapy. If the subjects relapsed, a new quit date was established, the relapse situation was reviewed to determine its causes, and strategies were developed to overcome similar situations. Two weeks after each session, the subjects were contacted by telephone to assess their smoking and to provide reinforcement for nonsmoking. Subjects who had relapsed were scheduled for a face-to-face session as soon as possible. Each telephone contact was approximately 5–10 minutes long; thus, approximately 40 to 80 minutes of telephone counseling were provided.
Counselors were trained in a multistep process. After reading the treatment manual, they observed the supervisor (G.L.H.) providing the treatment intervention to two cohorts. They were then observed by the supervisor as they treated a cohort. The same training procedure was used for telephone contacts. Counselors met weekly for supervision with G.L.H. and R.F.M. Brief and extended interventions are described in detail in manuals available from the first author.
Data Analysis
The planned group size was based on setting the power at 80%, with a type I error rate of 0.05. The estimated effect size was taken from Hall et al.
(2) and factored into the anticipated attrition rate. Logistic regression was used to test the effect of drug/placebo dose and treatment duration on repeat 7-day abstinence. A two-by-two design was used, with duration and dose as independent variables and the primary hypothesis evaluated by testing the dose-by-duration interaction. A generalized estimating equation, a generalization of the classic linear model that uses quasi-likelihood estimation, was used to test the hypotheses about point-prevalence abstinence at weeks 24, 36, and 52
(17,
18). SAS version 8.02 was used for all analyses
(19).
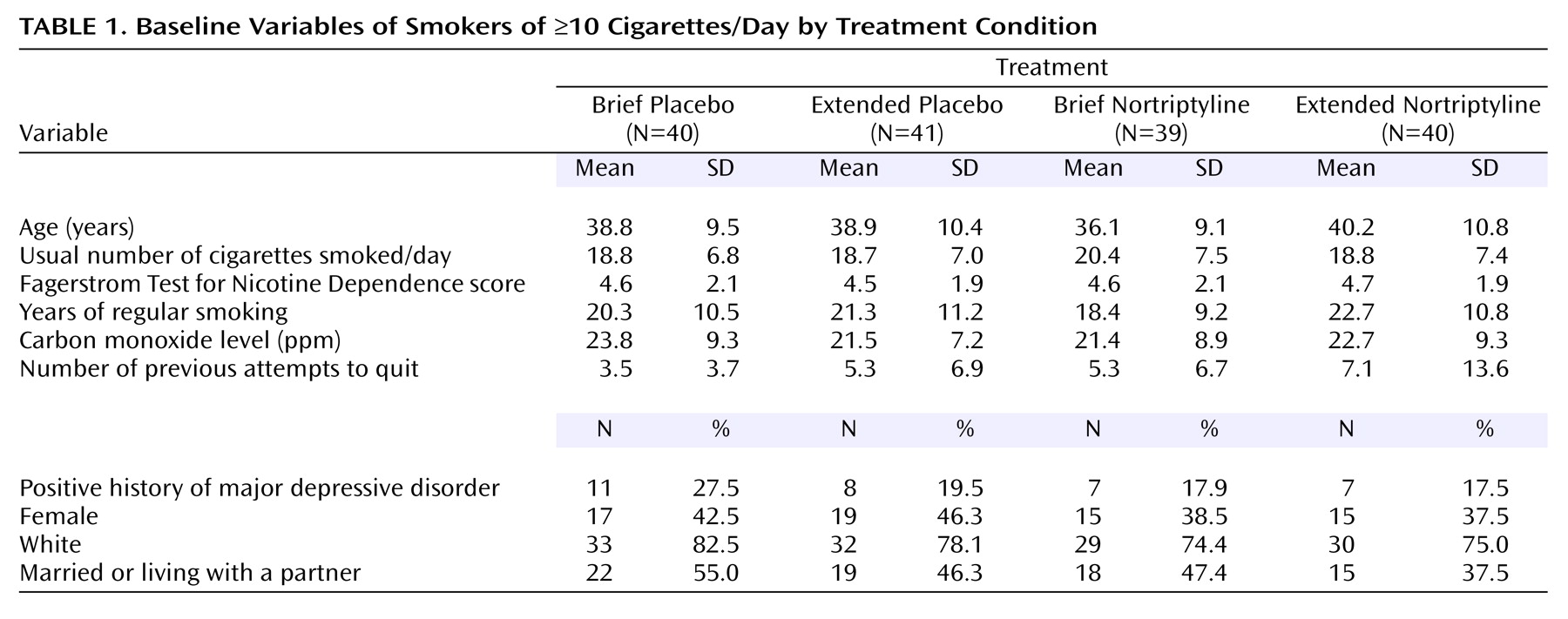
To ensure that randomization had not been compromised, we compared the four experimental cells on the baseline variables in
Table 1 using analysis of variance (ANOVA) for continuous variables and chi-squares analysis for categorical variables. To identify potential covariates, we correlated these baseline variables with point-prevalence abstinence at weeks 12, 24, 36, and 52 and with abstinence at all assessments versus no abstinence using point-biserial correlations. We included in preliminary hypothesis-testing models variables that had significant correlations with abstinence. These variables were eliminated if they did not contribute significantly to the final model. Determination of predictors of attrition from assessments was not useful because of the low attrition rates (week 12=2%, week 24=4%, week 36=14%, week 52=9%). To provide a comparison with earlier studies, we completed a chi-square test on active nortriptyline versus placebo at week 12.
Differences in weeks of medication/placebo dispensed were determined by a two-way ANOVA, with individual comparisons with the Tukey test if the overall F test proved significant. Differences in withdrawal symptoms between active and placebo conditions at week 12 were determined by repeated-measures ANOVA, with baseline level of symptoms used as a covariate to control for the general tendency to endorse such symptoms. Differences in side effects reported during the first 12 weeks of treatment were determined by comparing the number of subjects who reported the effect in the active and placebo conditions. During weeks 12–52, we included subjects in extended treatment only in the analyses of adverse events. For both sets of analyses, a series of chi-square tests—or Fisher’s exact test if the cell sizes were small—were used. All tests for all analyses described in this section were two-tailed.
Results
Subject Characteristics
Demographic, smoking, and psychiatric characteristics of the subjects in each experimental condition are shown in
Table 1. There were no significant differences among intervention conditions.
Attrition
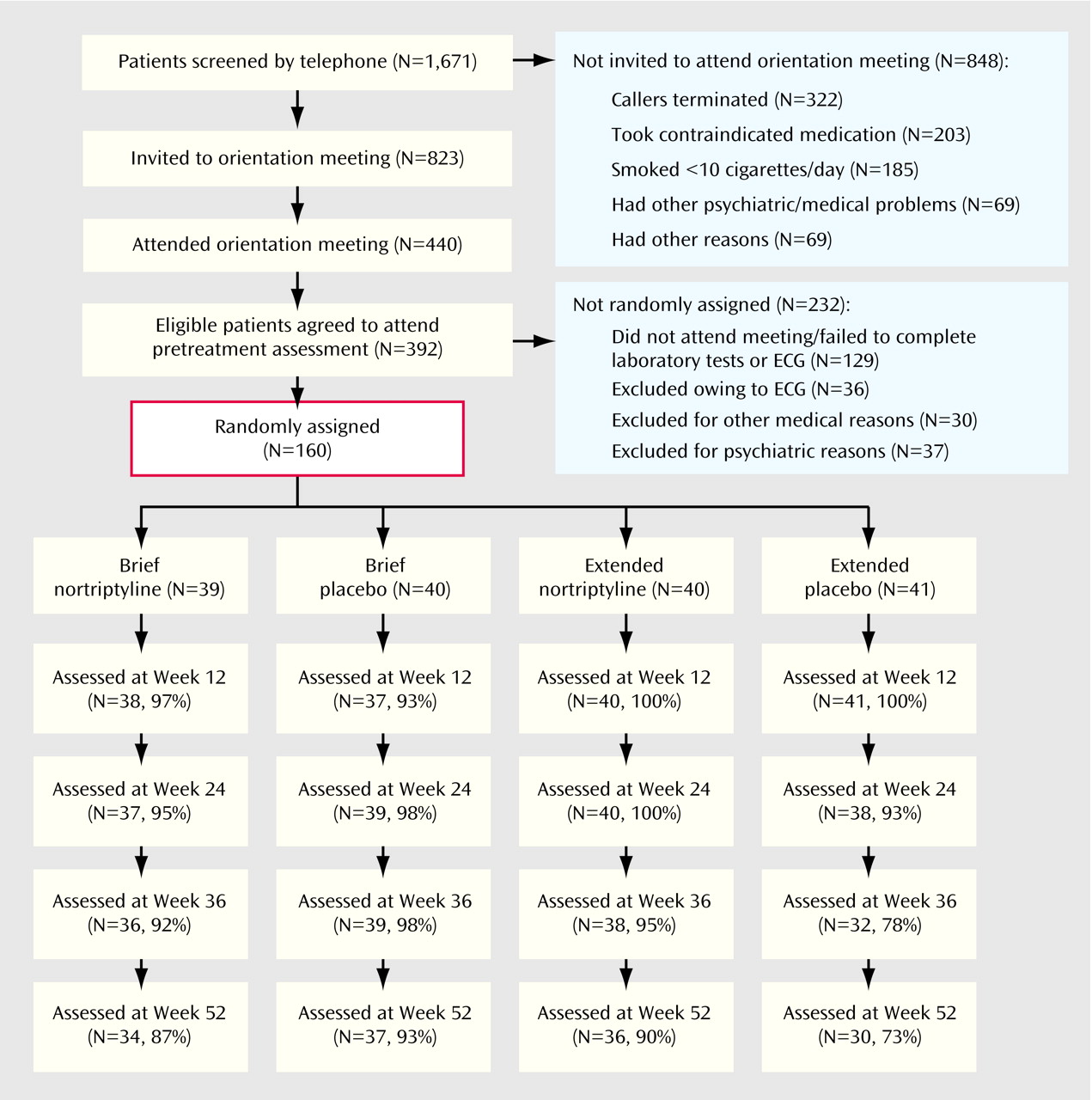
Figure 1 shows data flow from the first contact with the program to the week 52 assessment. Conditions did not differ on the percentage of subjects from whom we were able to collect smoking data at week 12 (df=3, 160, p=0.28, Fisher’s exact test), week 24 (df=3, 160, p=0.42, Fisher’s exact test), or week 52 (χ
2=7.40, df=3, 160, p=0.06). At week 36, there were significant differences in follow-up rates (df=3, 160, p=0.02, Fisher’s exact test); the attendance rate for the placebo extended condition was lower than for the three other conditions. Numbers and percentages of subjects contacted at each assessment are shown in
Figure 1.
The subjects were considered to have completed treatment in the brief conditions if they participated through week 12. In the extended conditions, treatment completion was attendance at six of nine sessions. There were no differences between active treatment and placebo in either the brief (active=30 of 39 [76.9%]; placebo=29 of 40 [72.5%]) (χ2=0.20, df=1, N=79, p=0.65) or the extended (active=27 of 40 [67.5%]; placebo=20 of 41 [48.8%]) (χ2=1.16, df=1, N=81, p=0.28) conditions.
Abstinence
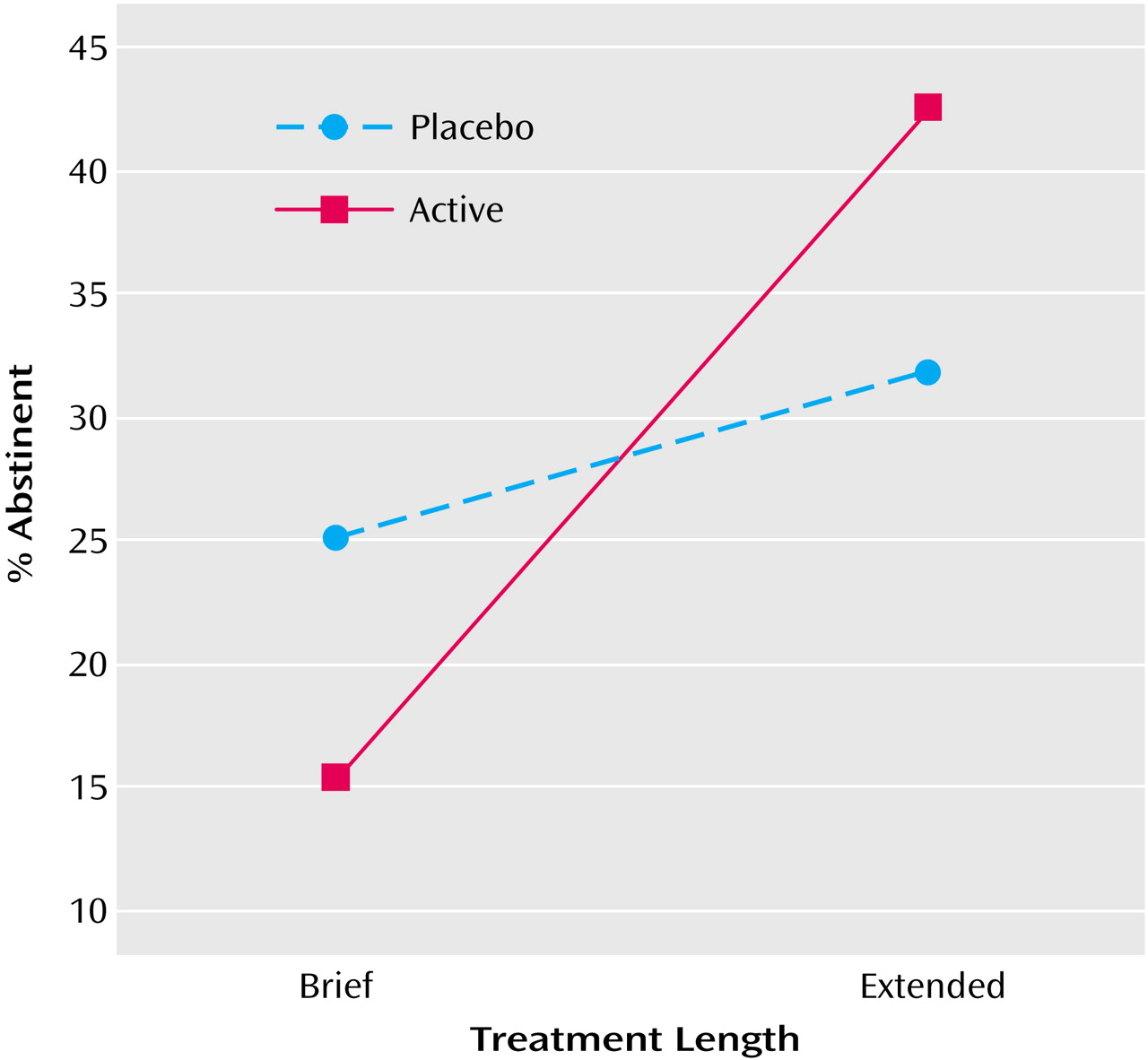
The primary hypothesis—that subjects in the active extended condition would be more likely to report repeated abstinence at each of weeks 24, 36, and 52 than subjects in the remaining three conditions—was confirmed by a logistic regression analysis testing the significance of the dose-by-duration interaction. The duration-by-dose interaction was significant (χ
2=6.90, df=1, N=160, p=0.009), as was the main effect of major depressive disorder (χ
2=5.02, df=1, N=160, p=0.03); a positive history for major depressive disorder predicted abstinence. As
Figure 2 indicates, the effect of extended duration on obtaining consistent abstinence was greater for the subjects receiving active drug than for those receiving placebo.
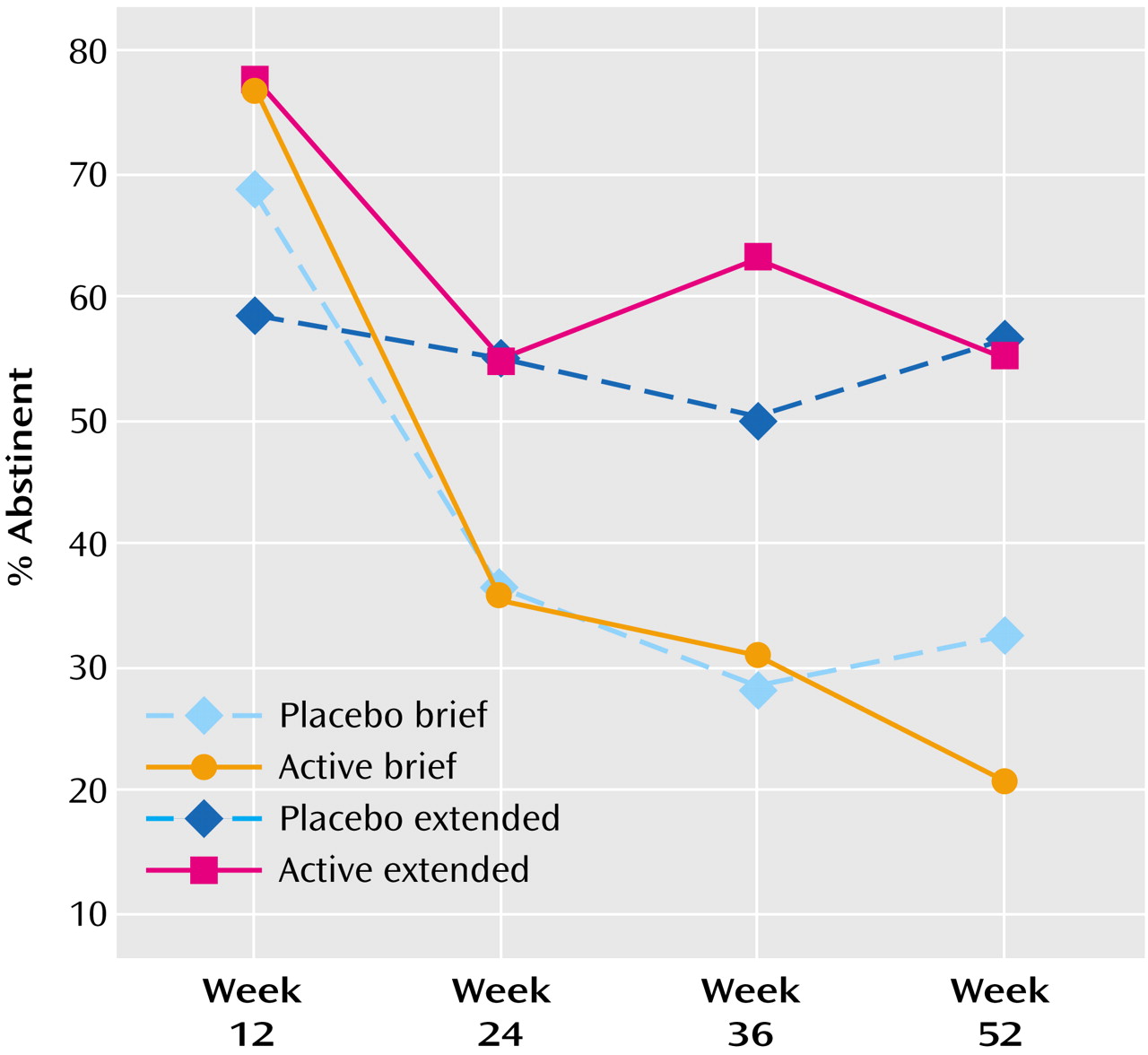
To test the second hypothesis, we computed a generalized estimating equation analysis with missing data omitted from the model, as is currently recommended for longitudinal analyses
(20,
21). The duration-by-dose-by-time-of-assessment was significant (χ
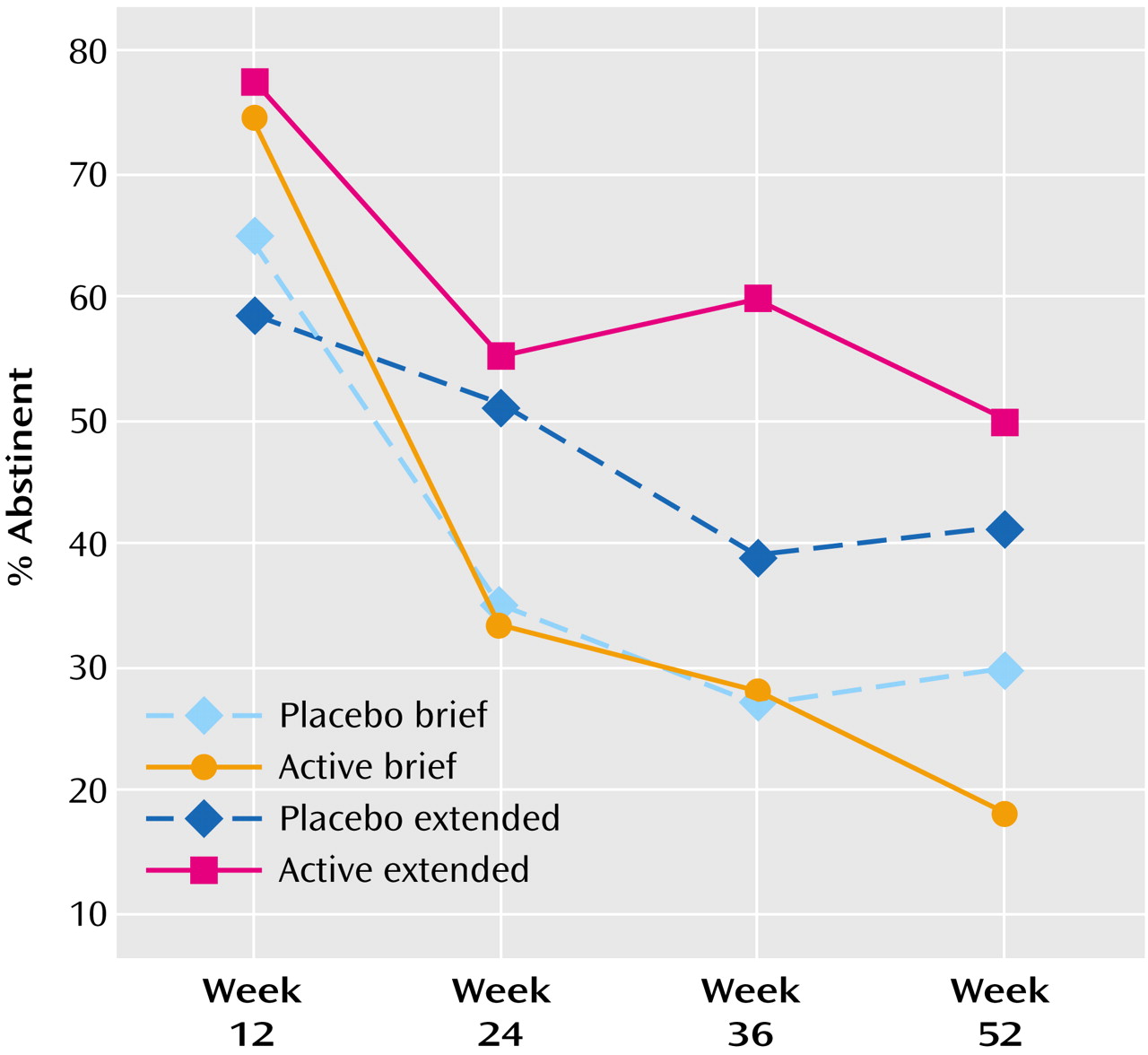
2=11.90, df=3, p=0.008, N=160). Comparison of the parameters estimates indicated that the active brief condition differed significantly from the placebo extended condition (odds ratio=0.47, 95% confidence interval [CI]=0.30–0.75, p=0.001) as did the placebo brief condition (odds ratio=0.69, 96% CI=0.49–0.95, p=0.02), but the extended active condition did not differ significantly from the extended placebo condition (odds ratio=0.81, 95% CI=0.60–1.09, p=0.17). In this model, a history of major depressive disorder did not produce main effects or interact with treatment condition. Replication of the generalized estimating equation with missing data coded as “smoking” did not alter the significance levels markedly. Abstinence rates by condition at each assessment are shown in
Figure 3 (missing data=missing data) and
Figure 4 (missing data=smoking).
As in earlier studies, active drug produced higher abstinence rate than placebo at week 12, whether missing data was omitted (χ2=4.91, df=1, N=157, p<0.03, odds ratio=2.29, 95% CI=1.10–4.80) or coded as smoking (χ2=5.05, df=1, N=160, p<0.03, odds ratio=2.27, 95% CI=1.10–4.70).
Nortriptyline Adherence
There were differences among the four experimental cells during the last week that nortriptyline was dispensed to the participants (F=11.5, df=3, 156, p<0.0001). As would be expected, the two brief conditions differed significantly from the two extended conditions (p<0.007, Tukey’s test). Tukey’s test indicated that there were no differences between active drug and placebo in the brief conditions (placebo: mean=9.45 weeks, SD=3.50; active drug: mean=8.87, SD=3.75) or between active drug and placebo in the extended conditions (placebo: mean=18.48, SD=15.34; active drug: mean=21.15, SD=16.56).
As expected, there were no significant differences between the subjects in brief and extended active nortriptyline treatment on nortriptyline blood levels at week 6: brief subjects, mean=58.9 (SD=34.3); extended subjects, mean=69.3 (SD=60.1) (t<10, df=56.3, p=n.s.).
Withdrawal Symptoms
The repeated-measures ANOVA indicated significant differences between drug conditions at baseline (F=5.92, df=1, 94, p<0.02) (active drug: mean=7.32, SD=5.76; placebo: mean=4.83, SD=4.10). With baseline levels as a covariate, the differences at 12 weeks were significant (F=3.93, df=1, 94, p=0.05) (active drug: mean=4.84, SD=4.60; placebo: mean=6.45, SD=5.14). Since subjects were smoking at baseline, differences at that point reflected a tendency to endorse symptoms. Mean symptoms in the placebo conditions increased from 4.8 to 6.5 from baseline to 12 weeks; the symptoms of those in the active drug conditions decreased from a mean of 7.3 to 4.8.
Side Effects
During weeks 0–12, subjects in the active drug conditions were more likely to endorse seven of the 22 possible side effects than were the subjects given placebo. These effects were dry mouth (85% versus 40%), lightheadedness (44% versus 22%), shaky hands (30% versus 14%), constipation (38% versus 15%), blurry vision (23% versus 7%), difficulty urinating (13% versus 2%), and sexual difficulties (19% versus 2%) (p<0.01 for all comparisons except difficulty urinating, which was p<0.02). During weeks 12–52, for subjects only in extended treatment conditions, active drug subjects were more likely to report skin redness (2.5% versus 0%) (df=1, p=0.03, Fisher’s exact test) and sexual difficulties (8.9% versus 1.2%) (df=1, p=0.03, Fisher’s exact test) than were placebo subjects. There were no severe adverse events.
Fourteen subjects receiving active drug terminated medication because of side effects before the end of their respective treatment period (four in active brief treatment, 10 in active extended treatment); nine subjects receiving placebo terminated treatment because of side effects (five in placebo brief treatment, four in placebo extended treatment) (active versus placebo: p=0.18, Fisher’s exact test).
Knowledge of Drug
As part of the informed consent procedures, the participants were informed about the side effects of each drug. It is not surprising that the participants given active drug were more likely to guess that they had received active drug (63%) than the placebo participants were to believe they were taking active drug (37%) (df=1, p<0.0001, Fisher’s exact test, odds ratio=5.75, 95% CI=2.45–13.49).
Extra Study Medication in Abstinent Subjects
Reports of use of extra study medication were rare among abstinent subjects and did not greatly affect outcome. As suggested by a recent consensus paper
(22), we included individuals using such medication as treatment successes. At week 24, two abstinent subjects reported using extra study medication (one placebo extended subject and one active extended subject used nicotine-replacement therapy), and at week 36, two abstinent subjects reported using nicotine-replacement therapy (one placebo extended subject and one active brief subject). One abstinent subject at week 36 (in the active extended condition) reported using a combination of nicotine-replacement therapy and bupropion. At week 52, two abstinent subjects reported using nicotine-replacement therapy (both in the placebo extended condition), and one abstinent subject (in the placebo brief condition) reported using bupropion.
Discussion
This study represents a new model of intervention in which treatment is viewed not as a one-time event or a few brief sessions but as an ongoing process over an extended time period as befitting a chronic disease. The combination of pharmacological and psychological intervention produced some of the highest abstinence rates reported in the literature. The 1-year point-prevalence rate of 50% in the active extended condition is unparalleled, with the only comparable rates having been reported over approximately 20 years ago in an uncontrolled trial of rapid smoking
(23,
24). There are, of course, factors that might have contributed to this rate, other than the interventions. These include low to moderate dependence, as evidenced by the Fagerstrom Test for Nicotine Dependence scores and self-selection into the trial.
The intervention used in the current study differs from others reported in the literature in that it not only combines modalities, but it provides them for an extended time period, independent of the subjects’ abstinence status over that time period. Analyses of point-prevalence data suggest that extended counseling with placebo does not differ significantly from extended counseling with active drug. Active drug plus extended counseling is superior in helping smokers achieve repeated abstinence, however. Since repeated abstinence is considered a better outcome than single point-prevalence outcome, we suggest that extended counseling is most important in attaining abstinence, but more reliable abstinence will be attained by combining drug and counseling.
Nortriptyline does not have a U.S. Food and Drug Administration indication for cigarette smoking. It would be interesting, therefore, to attempt to replicate the findings of the current study with sustained-release bupropion.
There were differences between active drug and placebo in the number of side effects during the first 12 weeks of treatment on seven of 22 possible symptoms, including sexual difficulties, that were reported by 20% of the subjects receiving nortriptyline. During the extended treatment period, there were fewer differences between the subjects in the active and placebo conditions, and the actual number of participants reporting them was low in all conditions. The number and pattern of side effects experienced is worth noting, however, and may be a barrier to nortriptyline use for some. Nortriptyline is an inexpensive generic drug that can be used safely with many smokers, and extended treatment with it may provide additional aid in maintaining abstinence.
High abstinence rates achieved at week 12, which we believe reflect the combination of two pharmacological treatments, nortriptyline with nicotine-replacement therapy and psychological intervention, contributed to the high overall rate; extended treatment appears to have reduced the rate of relapse.
We also attribute the high abstinence rates obtained to the use of psychological counseling and regular phone contacts. The extent to which this level of counseling is necessary or cost-effective has not been studied. Although some might argue that the expense and time of extended treatments would preclude widespread implementation, even a relatively expensive smoking treatment is likely to be among the most cost-effective of medical interventions, given the high health care costs of tobacco use
(25). Data such as these may show chronic smokers and their providers that such an effort is needed for successful cessation.
One question raised by these data is whether the differences observed are maintained in a longer follow-up period. A second question, more relevant to a chronic disease model, is whether extended treatment would continue to have beneficial effects when provided over a more prolonged period or indefinitely.
A history of major depressive disorder correlated significantly with consistent abstinence. The magnitude of the correlation was small (–0.15), however. This may represent a chance finding.
There were significant differences between nortriptyline and placebo at week 12, but inspection of the data suggested that they did not endure. This is in contrast to earlier findings by our group and others indicating a significant effect on point-prevalence abstinence for nortriptyline
(2–
4). Psychological intervention during the brief treatment period was comparable in the three studies. It is possible that the use of the nicotine patch swamped the effects due to nortriptyline, but if so, it is puzzling that the effect was not evident at week 12, immediately after termination of nicotine-replacement therapy.
It can be argued that many smokers may not be willing to engage in such intensive, long-term treatment. We propose that publication of data such as these may be needed to convince practitioners (and smokers) that substantial abstinence rates can be achieved, but treatment commensurate with tobacco’s addictive properties is required. Thus “generalizability” may be a moving target that can only be assessed after results such as these are widely disseminated.
This study is unusual in that the results of blinding were reported. When they are reported, however, it is not unusual for subjects to be able to guess their treatments. This is probably the rule, rather than the exception for behaviorally active drugs
(2,
4,
26).