Neuroimaging studies have indicated that training over brief periods leads to a decreased activation of cortical regions related to working memory
(1–
3). However, the length of training was restricted to hours in order to complete the respective functional magnetic resonance imaging (fMRI) experiments on a single day. In contrast to decreased activation in efficiently learned processes, previous studies have demonstrated that increased activation corresponds to better performance during working memory tasks
(4,
5). These results appear to be rather conflicting but may be adequately addressed by investigating training-related activation changes over longer periods.
This is of particular importance since the underlying mechanisms for memory processes on the neuronal level—repetition suppression and enhancement
(6)—may depend on training history, in particular, individual base level, or training intensity. Moreover, two clinical studies
(7,
8) have described a compensatory increase in cortical activation related to use of working memory in patients with schizophrenia after training over 6 to 12 weeks.
Therefore, in the present study, we examined cerebral activation changes over a 4-week training program. To test the hypothesis that cerebral activation follows a complex time course, with initial activation increases at the time of improved performance but subsequent decreases after consolidation of performance gains, healthy subjects were investigated during three sessions (at baseline and after 2 weeks and 4 weeks of training, respectively) while executing a visual spatial working memory task by using fMRI.
Method
Nine right-handed healthy male subjects (ages 26 to 32 years, with 4 to 6 years of university-level education) were included. The subjects were medication free, task naive, and had no history of drug abuse, head trauma, or neurological or psychiatric illness. All subjects gave written informed consent, according to institutional guidelines (the Ethics Committee of the University of Heidelberg).
The spatial variant of the n-back task used was already applied in several previous fMRI experiments
(3,
9). The task is comprised of three conditions (0-back, 1-back, and 2-back, respectively) with increasing difficulty, each consisting of 16 stimuli presented in a randomized sequence over a total duration of 45 seconds. The responses were continuously recorded to allow analyses of percent-correct replies. For the training, the subjects were asked to practice the task on a personal computer twice daily between the fMRI examinations.
A blocked design with four repetitions of each condition was performed at 1.5 T (Magnetom Vision Plus, Siemens, Erlangen, Germany) by using the standard head coil and a gradient-echo prepared echo planar imaging sequence (volume of 15 slices, field of view=240×240 mm
2, voxel size=1.88×1.88×4 mm
3, flip angle=90°). The slice orientation was parallel to the anterior commissure/posterior commissure plane. Image processing and analysis was performed with SPM 99 software (http://www.fil.ion. ucl.ac.uk/spm/). Functional volumes were realigned to correct for motion artifacts, stereotactically normalized, and smoothed with a Gaussian kernel (full width at half maximum=8 mm). Statistical analysis was performed for each condition according to the general linear model approach
(10).
At the first level of analysis, a statistical model for each session and each subject was computed to contrast periods during which the 0-back condition was performed with the 1-back and 2-back conditions, respectively. To test our hypothesis, a volume-of-interest analysis was performed for each subject and condition by using the regions identified by the comparison of 2-back versus 0-back over all sessions (k=20 voxels; p<0.05, corrected for multiple comparisons). On the basis of a second-level analysis, all conditions were compared with each other by calculating analyses of variance with testing for linear and quadratic trends (SPSS for Windows, version 11.0.1, SPSS Inc., Chicago).
Results
Even in the first session, the subjects made only a few errors during the 1-back condition (mean rate of relative errors=1.7%, SD=2.3); the improvement after training (second session: mean=1.4%, SD=3.2, third session: mean=0.5%, SD=1.1) did not reach statistical significance (F=1.54, df=1, 8, n.s., linear contrasts). For the 2-back condition, the relative error rate improved significantly (F=10.1, df=1, 8, p≤0.05, linear trend) from a mean of 6.9% (SD=4.1) in the first session to 1.3% (SD=2.6) in the second session and remained stable in the third session (mean=1.4%, SD=2.7). Hardly any errors were recorded in the 0-back condition (first session: 0.2%, SD=0.6; second and third sessions: 0%, SD=0).
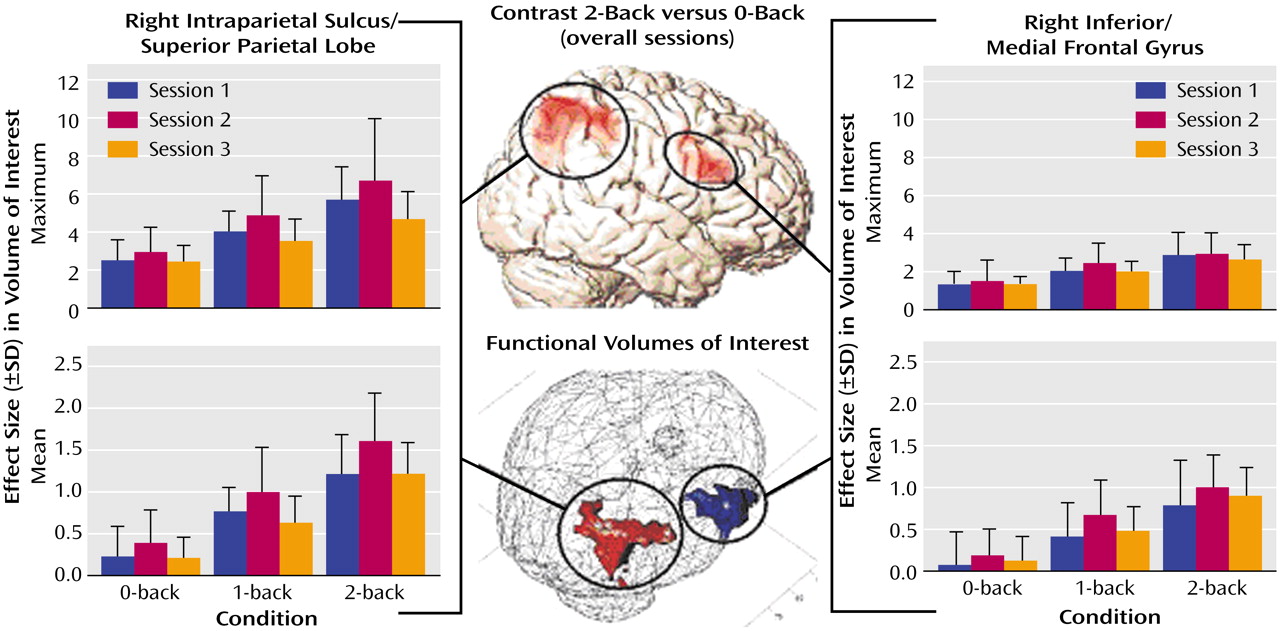
With respect to the cortical hemodynamic responses, the group analysis revealed two main activation clusters for the contrast comparing the 2-back versus 0-back tasks: the right inferior frontal gyrus (Brodmann’s area 45) and the right intraparietal sulcus (Brodmann’s area 39/40). Apart from these regions, the respective functional volumes of interest also included parts of the medial frontal gyrus (Brodmann’s area 9/46) and the superior parietal lobe (Brodmann’s area 40). As presented in
Figure 1, the changes in effect sizes were used to quantify the impact of training. With respect to the intraparietal sulcus/superior parietal lobe, a significant quadratic trend was found for the mean effect sizes under the 1-back condition (F=6.50, df=1, 8, p<0.05) and under the 2-back condition (F=9.96, df=1, 8, p<0.01). The respective values under the 0-back condition did not reach significance (F=4.37, df=1, 8, p=0.07). In the inferior/medial frontal gyrus, only a nonsignificant trend toward a quadratic function was found for the mean effect size under the 1-back condition (F=4.24, df=1, 8, p=0.07). Repeated analyses of the maximum effect sizes only revealed nonsignificant trends (3.37<F<4.43, df=1, 8, 0.07<p<0.10) toward quadratic functions for the intraparietal sulcus/superior parietal lobe
The mean and maximum effect sizes of both regions showed a significant (34.5<F<104.7, df=1, 8, 0.0001<p<0.001, linear trend) load effect with higher activation values at increased task demands.
Discussion
The brain regions activated in all sessions during performance of the visual spatial working memory task were generally consistent with previous reports and included right prefrontal and parietal areas
(9,
11,
12). In each session, higher task demands corresponded to activation increases in the respective regions. Similar load effects were described for a variety of tasks, including working memory, repetitive movements, and affect recognition
(9,
13,
14).
The training-related activation changes in the inferior frontal gyrus and the intraparietal sulcus are best described by an inverse U-shaped quadratic function with a negative exponent. While this function reached the significance level for the right intraparietal sulcus/superior parietal lobe under the 1-back and 2-back conditions only, both regions showed initial activation increases with improved performance, followed by subsequent activation decreases with consolidation of performance gains.
These activation changes support the hypothesis that training of working memory is mediated by different neuronal mechanisms, depending on training history. According to Desimone
(6), three neuronal effects in memory-demanding tasks are commonly observed, namely, repetition suppression, enhancement, and delay activity. Following this hypothesis, our results suggest that the prefrontal and parietal cortices may contain at least two parallel mechanisms mediating the training effects of working memory: an enhancement mechanism for active working memory and a suppressive mechanism that is engaged automatically by stimulus repetition. Training, therefore, may lead to a change in predominance of one of these mechanisms triggered by the ongoing requests on manipulation, storage, and attention.
Our study has potential implications for fMRI studies since training effects occur regularly whenever similar tasks are repeatedly given. At this point, the question of whether the inverse U-shaped course of cerebral activation changes also applies to other cognitive domains or may be modified by important individual characteristics, such as age, education level, or gender cannot be addressed. However, our results emphasize the prospect of investigating cerebral mechanisms underlying training effects and optimizing the respective programs.