Benzodiazepine hypnotics, which have been used widely since the early 1960s, are known to act by agonistic modulation of γ-aminobutyric acid A (GABA
A) receptor subtypes. However, the pharmacological actions mediated by the GABA
A receptor subtypes are still a matter of dispute
(1). Therefore, many questions regarding the specific drug actions of the benzodiazepine hypnotics remain to be answered, including the question of which neuroanatomical sites are affected by these drugs.
Positron emission tomography (PET) has been used effectively to visualize the functional neuroanatomy of human sleep, and oxygen-15 water ([
15O]H
2O) PET, which is well suited to the investigation of sleep states of relatively short duration, has revealed changes in regional cerebral blood flow (rCBF) associated with human non-REM and REM sleep
(2). The effects on human sleep of hypnotics acting on the GABA
A receptor, including the newly developed nonbenzodiazepines, have also been explored by using PET
(3). Unfortunately, previous studies failed to detect specific neuroanatomical sites for the actions of these hypnotics during sleep, possibly because of methodological problems. Therefore, we conducted a double-blind, crossover study of [
15O]H
2O PET with statistical parametric mapping to compare rCBF during human non-REM sleep after administration of triazolam or placebo.
Method
Fifteen healthy, right-handed, male university students (mean age=21.3 years, SD=1.0, range=20–23) served as study subjects. All gave written informed consent before participating in the study, which was approved by the Intramural Research Board of the National Center of Neurology and Psychiatry.
A maximum of eight intravenous injections of [15O]H2O were given to each subject during periods of wakefulness and light and deep non-REM sleep under continuous polygraphic monitoring on each experimental night of triazolam or placebo administration. The experiments were separated by a 1-week interval and did not include sleep deprivation. On the night of the experiment, electrodes were attached for polygraphy, and each subject lay face up on a scanner couch, with the head stabilized by an individually molded thermoplastic face mask secured to a plastic headholder. The headholder was fixed on the end of the scanner couch so that the head was protruding fully into the scanner field of view; the head was angled so that each subject’s canthomeatal line was parallel to axial planes of the PET scanner.
After two PET scans for wakefulness were obtained in the eye-closed condition, the subject took a capsule containing either 0.25 mg triazolam or placebo at 10:00 p.m. The lights were turned out at 10:30 p.m., and three scans for light non-REM sleep (stage 2 sleep) and three for deep non-REM sleep (stages 3 and 4 sleep, slow wave sleep) were performed between 11:00 p.m. and 2:00 a.m., when the polygrams showed typical patterns for these sleep stages.
EEGs were recorded from disc electrodes placed at F3, F4, C3, C4, Fz, and Cz; A1 plus A2 were used for reference. Monopolar electro-oculograms were recorded from both canthi, and bipolar electromyograms were recorded from the chin. Details of the polygraphic methodology are the same as in our previous study
(4). Sleep stage scoring was carried out according to the standardized sleep manual of Rechtschaffen and Kales
(5). Final evaluation of sleep stage scoring for each 90-second period during PET scanning was confirmed later by using C3 recording.
PET data were acquired with the use of a Siemens ECAT EXACT HR 961 scanner in the three-dimensional mode. The camera, having an axial field of view of 150 mm, acquired data simultaneously from 47 consecutive axial planes, which cover the whole brain, including the cerebrum, cerebellum, and brainstem. A spatial resolution of 3.8×3.8×4.7 mm of full width at half maximum was obtained after back-projection and filtering. The reconstructed image was displayed on a 128×128×47-voxel matrix (voxel size=1.732×1.732×3.125 mm). Transmission scanning was carried out before acquisition of the emission data by using a retractable, rotating 68Ga/68Ge source with three rods.
For each PET scan, an intravenous bolus of 7-mCi [
15O]H
2O was automatically flushed over 15 seconds. Scanning was begun manually 1 second after the initial rise in head counts and was continued for 90 seconds. Arterial blood was sampled automatically throughout the scanning period with a flow-through radioactivity detector. Absolute CBF was quantified by using the autoradiographic technique
(6,
7). Details of the PET data analysis are the same as in our previous study
(8).
After the appropriate design matrix was specified, estimates of the subject and condition were determined according to a general linear model at each and every voxel. Parameter estimates were compared by using linear contrasts. The contrast of interest in this article was the main effect of the drug during non-REM sleep. These analyses generated statistical parametric maps that were subsequently transformed to the unit normal distribution. The exact level of significance of volumes of difference between conditions was characterized by peak amplitude. Clusters of voxels that had a peak z score greater than 3.09 (threshold p<0.001) were considered to show significant difference. A corrected p value of 0.05 was used as a statistical cluster threshold.
Results
We analyzed the PET data of nine of the 15 subjects, who provided us with complete sets of data during periods of wakefulness and light and deep non-REM sleep on the nights of triazolam and placebo administration. One-way analysis of variance showed no significant difference in the absolute values for global CBF during light or deep non-REM sleep between the placebo and triazolam conditions. Mean absolute global CBF during light non-REM sleep with placebo was 30.4 ml/100 g per minute (SD=5.1), and that with triazolam was 28.8 ml/100 g per minute (SD=4.6) (F=0.51, p=0.49). Mean absolute global CBF during deep non-REM sleep with placebo was 30.6 ml/100 g per minute (SD=3.2), and that with triazolam was 29.4 ml/100 g per minute (SD=3.1) (F=0.64, p=0.44).
When there are no differences in the absolute values for global CBF, comparison of normalized values for regional CBF is often more sensitive for detection of differences than comparison of absolute values and reflects real changes in rCBF
(2,
8). Therefore, normalized rCBF values obtained for non-REM sleep with placebo were compared with those obtained with triazolam by analysis of covariance for the nine subjects who provided complete sets of data.
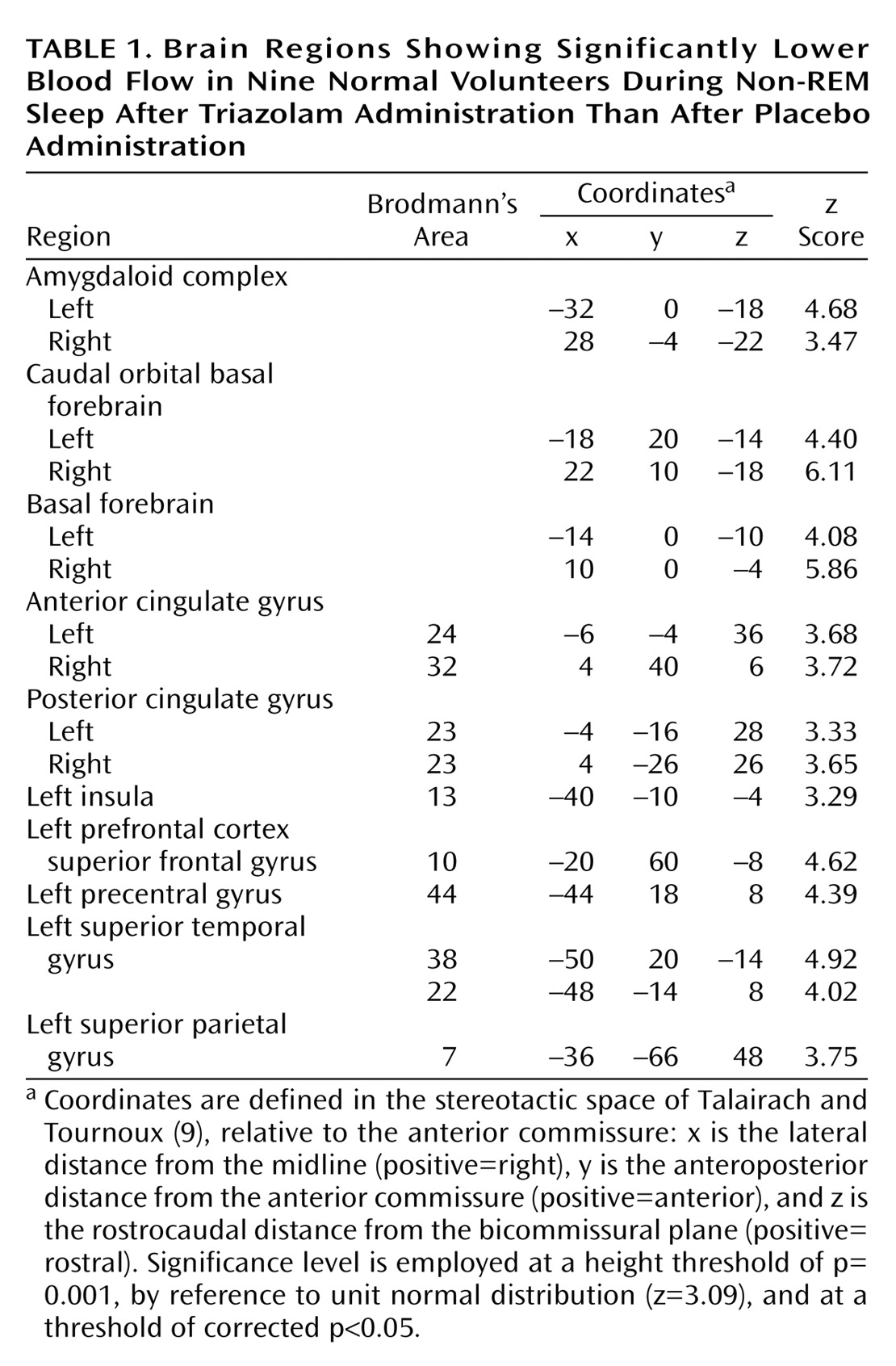
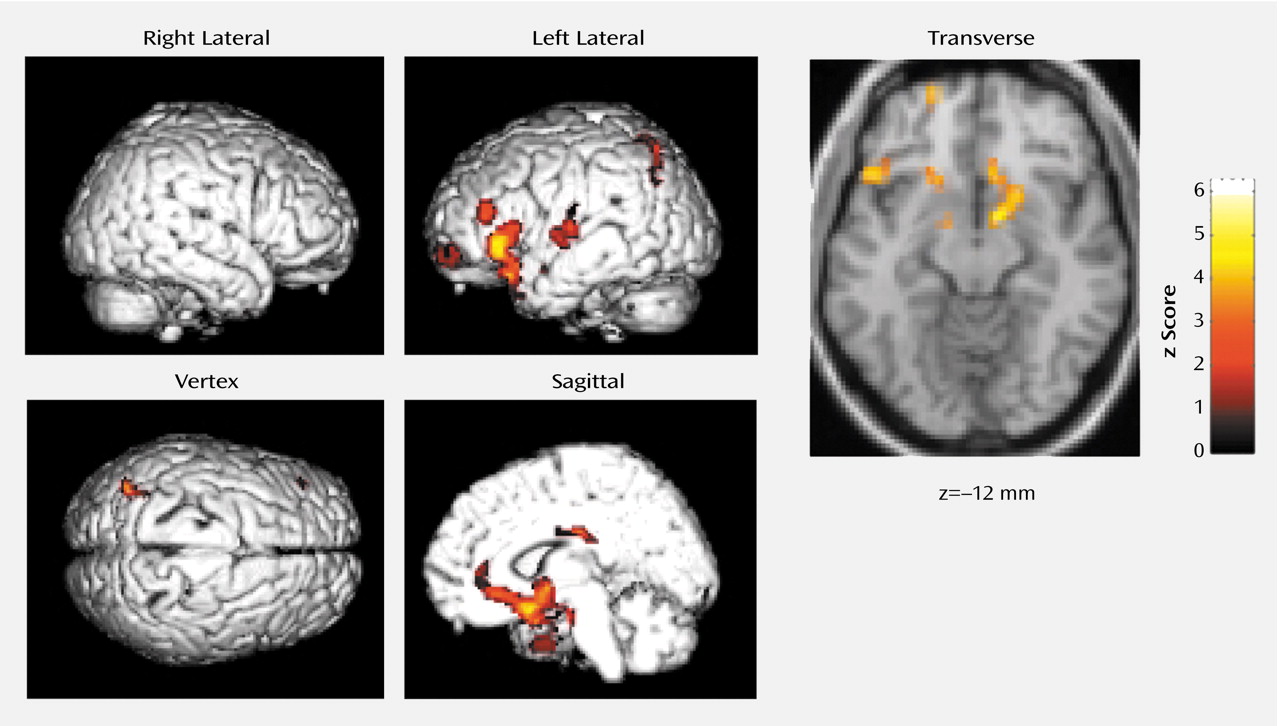
Blood flow was lower in the basal forebrain, amygdaloid complexes, anterior (Brodmann’s area 32, 24) and posterior (Brodmann’s area 23) cingulate gyri, and the left neocortical regions, including the superior temporal gyrus (Brodmann’s area 38, 22), precentral gyrus (Brodmann’s area 44), superior frontal gyrus (prefrontal cortex) (Brodmann’s area 10), superior parietal gyrus (Brodmann’s area 7), and insula (Brodmann’s area 13), during non-REM sleep when triazolam was given than when placebo was given (
Table 1 and
Figure 1). There was no significant increase in rCBF of any region during non-REM sleep under triazolam administration in comparison to that under placebo administration.
Discussion
Using functional neuroanatomical PET, we found that the basal forebrain and the amygdaloid complexes of human subjects showed deactivation during non-REM sleep after triazolam treatment. Wakefulness is known to be maintained by multiple neuronal populations spread out from the metencephalon to the diencephalon, the mesencephalic reticular formation, posterior and posterolateral hypothalamus, and basal forebrain cycle
(10). Our data show that triazolam has its main impact on the basal forebrain rather than on the other wake-promoting structures. Therefore, the hypnotic effect of the benzodiazepines may result mainly from inhibition of the forebrain control system for wakefulness. This is supported by the finding in our previous study
(8) that the basal forebrain is deactivated during deep non-REM sleep in normal humans, suggesting that deactivation of the basal forebrain is involved in the non-REM sleep networks. Additionally, our present finding that triazolam deactivated the amygdaloid complexes, which are involved in emotional response, including anxiety and fear, during non-REM sleep, suggests that the anxiolytic effect of the benzodiazepines is also associated with their hypnotic effect.
Most benzodiazepines, including triazolam, induce particular changes in EEG activity and sleep stages. They decrease sleep latency and night awakenings, but, in terms of sleep architecture, they reduce slow wave sleep and REM sleep. Thus, the benzodiazepines have been mysterious agents with a paradoxical effect, and the mechanism explaining this effect has remained unclear. The present finding that triazolam deactivates the basal forebrain may account for the paradoxical effect. The basal forebrain is structurally and functionally heterogeneous. Since the basal forebrain contains the type of tonic neurons that are active specifically in wakefulness and the type that are active specifically in sleep
(10), and since the latter type of neurons are particularly related to the induction of slow wave sleep
(11), relatively diffuse deactivation of this region might facilitate initiation of sleep but inhibit slow wave sleep.
The left neocortical regions, including the superior temporal gyrus, precentral gyrus, superior frontal gyrus (prefrontal cortex), and superior parietal gyrus, were deactivated after triazolam administration. Asymmetric changes in rCBF during the sedative state induced by midazolam, a short-acting benzodiazepine, have been reported
(12): midazolam was shown to decrease rCBF in the left prefrontal cortex in a dose-dependent fashion. The deactivation of the left neocortical regions by triazolam in the present study is basically consistent with the finding of the midazolam study, suggesting that left-hand cortical areas are more sensitive to the modulation of benzodiazepines.