Schizophrenia patients have been repeatedly found to be impaired in the accurate perception of facial expressions
(1). Recently, the neurophysiological correlates of this dysfunction have been investigated by using functional magnetic resonance imaging
(2) as well as event-related potentials
(3). The event-related potential study revealed that schizophrenia patients exhibited reduced cortical activity during affect recognition within a 180–250-msec latency range. Processing of the general features of a face, which occurs earlier (at about 170 msec) and presumably involves the fusiform gyrus
(4–
6), is reflected by a negative event-related potential component (N170) over temporoparietal electrode positions
(4–
9).
Although reduced volumes of the fusiform gyrus were recently described in first-episode schizophrenia patients
(10), to our knowledge no published study exists showing reduced cortical activation during face processing in schizophrenia patients. In this study, we investigate the N170 component elicited by pictures of faces and by pictures of buildings (control stimuli). We hypothesized that schizophrenia patients would show less of a difference in N170 amplitude between the face and building pictures than would the healthy comparison subjects, indicating a dysfunction in face processing.
Method
Participants
Twenty-four patients with DSM-IV schizophrenia (mean age=32.2 years, SD=10.0, range=18–57) and 28 healthy comparison subjects (mean age=33.8 years, SD=13.0, range=18–57) participated in this study. Nineteen patients and 12 comparison subjects were male; all subjects were right-handed. Diagnoses were determined by a senior psychiatrist following a comprehensive psychopathological assessment and were independently confirmed by the first author. The level of education was similar in the patients and comparison subjects (10.3 and 10.1 years, respectively). After complete description of the study to the subjects, written informed consent was obtained. Seventeen patients were treated with neuroleptic medication (three with typical antipsychotics, eight with atypical antipsychotics, and six with both) receiving a mean of 666 mg/day in chlorpromazine equivalents (SD=430), with doses ranging from 150 to 1725 mg/day. Patients’ mean scores for negative symptoms, positive symptoms, and general psychopathology on the Positive and Negative Syndrome Scale were 18.6 (SD=6.4), 10.6 (SD=3.1), and 25.3 (SD=7.1), respectively.
Procedures
EEG recordings were obtained from 21 scalp electrodes positioned according to the international 10-20 system against linked mastoids. Three additional electrodes were placed at the outer canthi of both eyes and below the right eye to monitor eye blinks. The EEG was sampled continuously at a rate of 256 Hz with a bandpass from 0.1 to 70 Hz. Impedances did not exceed 5 kΩ .
The task consisted of two sets of black-and-white pictures with a similar mean gray value (faces: mean=132.3, SD=9.4; buildings: mean=131.5, SD=8.4), showing 12 different faces
(11) and 12 buildings, which were repeatedly presented three times each in a pseudo-randomized order (visual horizontal angle=4.6°, vertical angle=6.3°). Each stimulus was presented for 500 msec with an interstimulus interval of 1500 msec. Subjects were asked to classify silently every slide as a face or a building. After six to 12 stimuli, they were asked to report verbally the last classification.
Data Analysis
Epochs (–100 msec prestimulus to 500 msec) with amplitudes or voltage steps exceeding 98 μV were excluded from further analyses. After average reference calculation, the artifact-free trials (≥20 epochs) were averaged separately for each subject and condition. On the basis of grand mean curves, the face-specific N170 was detected as the maximum negative peak between 113 and 230 msec for the electrodes P7 and P8. Additionally, we analyzed the P1 (70–156 msec) and the P2 (160–281 msec) as positive peaks over occipital electrode positions (O1, O2). Peak amplitudes and latencies were statistically analyzed using a two-by-two-by-two (side [left, right] by condition [face, building] by group [comparison subjects, schizophrenia patients]) analysis of variance (ANOVA) with repeated measures for the factors side and condition. Greenhouse-Geisser correction was used for the degrees of freedom. Statistical analyses were calculated by using SPSS 11 (SPSS, Chicago).
Results
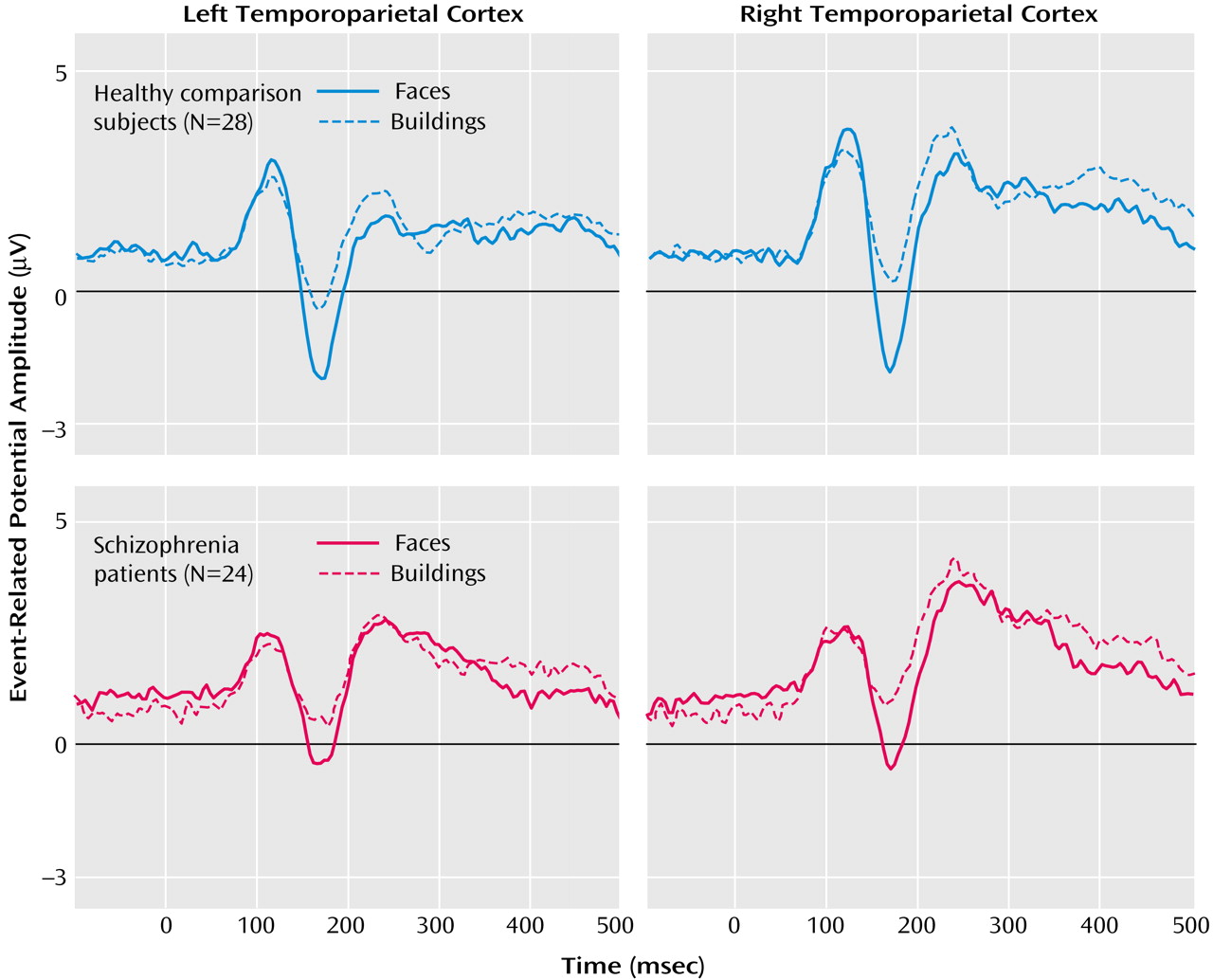
As seen in
Figure 1, schizophrenia patients and healthy comparison subjects clearly showed the N170 component over temporoparietal positions (electrodes P7 and P8). The statistical analysis for the N170 amplitudes revealed significant main effects for condition (F=127.7, df=1, 50, p<0.0001) and group (F=7.7, df=1, 50, p<0.01). Furthermore, significant condition-by-group (F=4.4, df=1, 50, p<0.05) and condition-by-side (F=4.1, df=1, 50, p<0.05) interaction effects occurred. To analyze these interactions in more detail, differences in amplitudes of the N170 to faces and buildings were calculated and further analyzed with a post hoc ANOVA with side (P7, P8) and group (comparison subjects, schizophrenia patients) as factors. The significant main effect of group (F=4.4, df=1, 50, p<0.05) indicated that schizophrenia patients had lower differences in event-related potential amplitudes for faces versus buildings than did the comparison subjects. The significant main effect of side (F=4.1, df=1, 50, p<0.05) indicated higher differences over the right compared with the left side. To analyze whether face processing was selectively impaired, we compared the N170 amplitudes between comparison subjects and patients separately for both conditions. Patients had significantly reduced N170 amplitudes for faces (P7: t=–2.8, df=50, p<0.01; P8: t=–2.7, df=50, p<0.01) but not, or only as a tendency, for buildings (P7: t=–1.6, df=50, n.s.; P8: t=–1.8, df=50, p<0.10).
For the healthy group, no sex differences occurred for the N170 amplitudes (condition by sex: F=0.22, df=1, 26, n.s.).
For the P1 amplitude, a significant main effect of condition occurred (F=24.3, df=1, 50, p<0.001), indicating higher amplitudes for faces (mean=5.9, SD=0.4) than for buildings (mean=5.2, SD=0.4). P2 amplitudes differed significantly between groups (main effect of group: F=4.7, df=1, 50, p<0.05), with higher amplitudes for patients (mean=6.8, SD=0.5) relative to comparison subjects (mean=5.3, SD=0.5). No significant condition-by-group interactions were found (P1: F=0.6, df=1, 50, n.s.; P2: F=0.5, df=1, 50, n.s.).
Neither N170 amplitudes nor the differences between faces and buildings correlated with medication in chlorpromazine equivalents or with Positive and Negative Syndrome Scale scores.
Discussion
Schizophrenia patients showed a specific reduction of the N170 component to faces relative to comparison subjects. As the N170 reflects the structural encoding of faces
(12), this finding indicates a specific deficit of schizophrenia patients in face processing. This result is in accordance with reports of reduced volumes of the fusiform gyrus
(10) and missing activation of the fusiform gyrus during facial emotion processing
(2) in patients with schizophrenia. Of most interest is that our event-related potential findings allow the conclusion that these disturbances in face processing occur already at about 165 msec after stimulus onset, much earlier than alterations of facial expression analyses, which were observed at about 180–250 msec in schizophrenia
(3). Regarding the impairments of facial expression analyses described for schizophrenia patients
(1), our results indicate that these deficits might be caused by a generally impaired early-stage visual processing
(13) and not by an emotional deficit. Our results might indicate that additional brain areas beside the frontal cortex are involved in the pathophysiology underlying schizophrenia diseases.