Disturbed neural circuits in schizophrenia, involving the prefrontal cortex, thalamus, and cerebellum, have been proposed as a basis for a “cognitive dysmetria”
(1) or poor mental coordination, which it is suggested, underlies the cognitive deficits and symptoms in schizophrenia. Functional studies have provided supportive evidence for disturbed neural networks. Consistent with a notion of dysconnectivity are the findings of low regional blood flow to the prefrontal cortex and greater than normal blood flow to the thalamus and cerebellum
(2–
4) and, in patients with negative symptoms, low right temporal and ventral prefrontal cortex metabolism and high cerebellar cortex metabolism
(5). Low structural volumes of the frontal and temporal lobes, thalamus, left cerebellum, and right vermis have been found in schizophrenic patients by using whole-brain deformation-based morphometry
(6). Findings from magnetic resonance imaging (MRI) of abnormalities in the mediodorsal thalamic nucleus, which has reciprocal connections with the prefrontal cortex, are consistent with the cognitive deficits seen in schizophrenia involving the frontal lobe
(7). Low limbic, thalamic, and cerebellar volumes in relatives of patients with schizophrenia suggest these may also be trait markers for schizophrenia
(8). In childhood-onset schizophrenia, the results of structural studies, which indicate a progressive loss of cerebellar volume
(9), low thalamic area
(10), and low anterior frontal volume
(11), and functional studies, which demonstrate hypofrontality
(12) and bilaterally higher cerebellar metabolism
(13), are compatible with a neurodevelopmental basis for a dysconnectivity syndrome.
It was decided to test the hypothesis that there is a developmental basis for a dysconnectivity syndrome in early-onset schizophrenia that is manifested as low volumes of the prefrontal cortex, thalamus, and cerebellum.
Method
Subjects
Data from 16 subjects with schizophrenia and 16 matched normal comparison subjects were available from an initial study
(14). The subjects were diagnosed at two times by using a semistructured interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children
(15), according to the DSM-III-R criteria for schizophrenia. The interviews were undertaken by experienced, trained child psychiatrists (A.C.J. and A.J.), and in addition all had independent clinical diagnoses at both times, which were in agreement with the research diagnoses. Symptoms were rated by using the Positive and Negative Syndrome Scale for schizophrenia
(16). The subjects with schizophrenia and comparison subjects with mental impairment (IQ <70) and those with histories of head injuries or neurological disorders, such as cerebral palsy, encephalitis, and epilepsy, were excluded. The comparison subjects were recruited from the community through their general practitioners and were screened for any history of emotional, behavioral, or medical problems. All of the subjects attended normal schools and came from the Oxfordshire region, which had a total population of 2.5 million. After complete description of the study, written informed consent was obtained (Oxford Psychiatric Research Ethics Committee study number 95/43). All of the patients with schizophrenia were receiving medication throughout the study; all 16 received atypical neuroleptics, and three were taking clozapine. The patients and comparison subjects received MRI brain scans at the two time points; the second was 2 to 3 years after the first on average
(17).
Imaging and Analysis
The subjects were scanned on a GE Signa 1.5-T MRI machine, which remained the same throughout the study period, with regular quality controls. The image sequences included a coronal volumetric T1-weighted radio-frequency-spoiled gradient echo (echo time=5 msec, repetition time=35 msec, flip angle=35°, field of view=20×20 cm, slice thickness=3 mm contiguous, acquisition matrix=256×256, slices=64) covering the whole brain with an inferior saturation band positioned below the base of the cerebellum.
All scans were analyzed by investigators blind to diagnosis (A.C.J., S.J., and A.J.). An image analysis program called RESCUE
(18) allowed regions of interest to be displayed in three-dimensional orthogonal views and to be outlined manually. Cross-sectional areas were then summated automatically. RESCUE uses a multiple hierarchical segmentation algorithm
(18). Calculations of edge boundaries are based on the gradient over a Gaussian scale and differences in mean luminosities of selected regions. The segmentation is semiautomatic with a manual parameter setting of white and gray matter.
Anatomical Markers
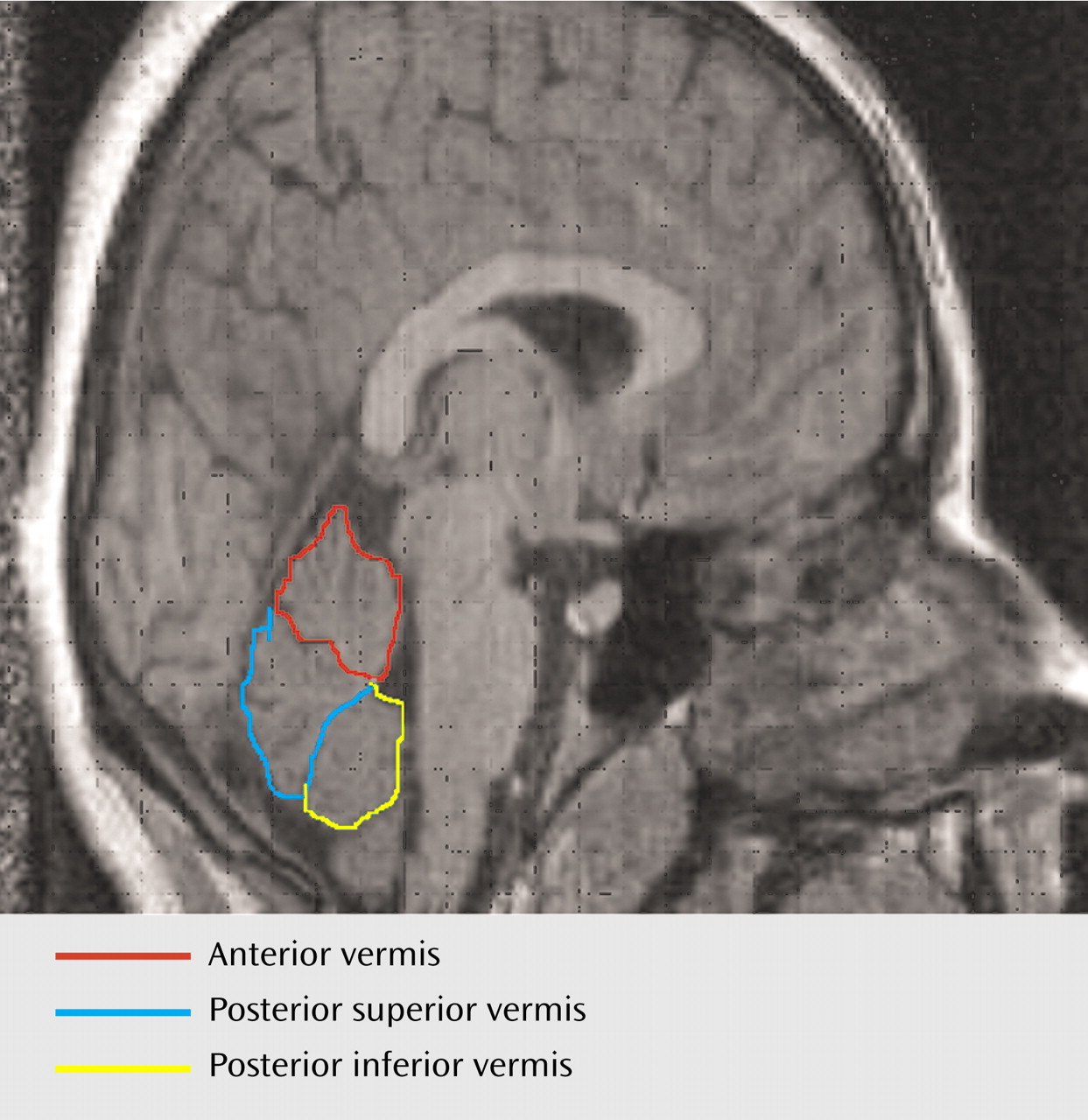
For the midcerebellar measurements, coronal scans were examined by using RESCUE. Measurements were displayed in three-dimensional orthogonal views to check correct midline alignment with respect to the cerebellum, which differed from the midcerebral slice in nearly one-half of the cases. Cerebellar and vermal volumes were outlined by using an atlas
(19) and according to a standardized method
(20). The midsagittal areas of the anterior, posterior superior, and posterior inferior lobes of the vermis were measured by manually tracing in the plane that bisected the pyramid of the vermis, the fourth ventricle, and the pyramid of the medulla (obex). The primary fissure and the fourth ventricle demarcated the anterior lobe (lobules I–V), the primary and prepyramidal fissures demarcated the posterior superior lobe (lobules VI and VII), while the posterior inferior lobe (lobules VIII–X) was demarcated by the prepyramidal fissure and the ventral edge of the fourth ventricle (
Figure 1).
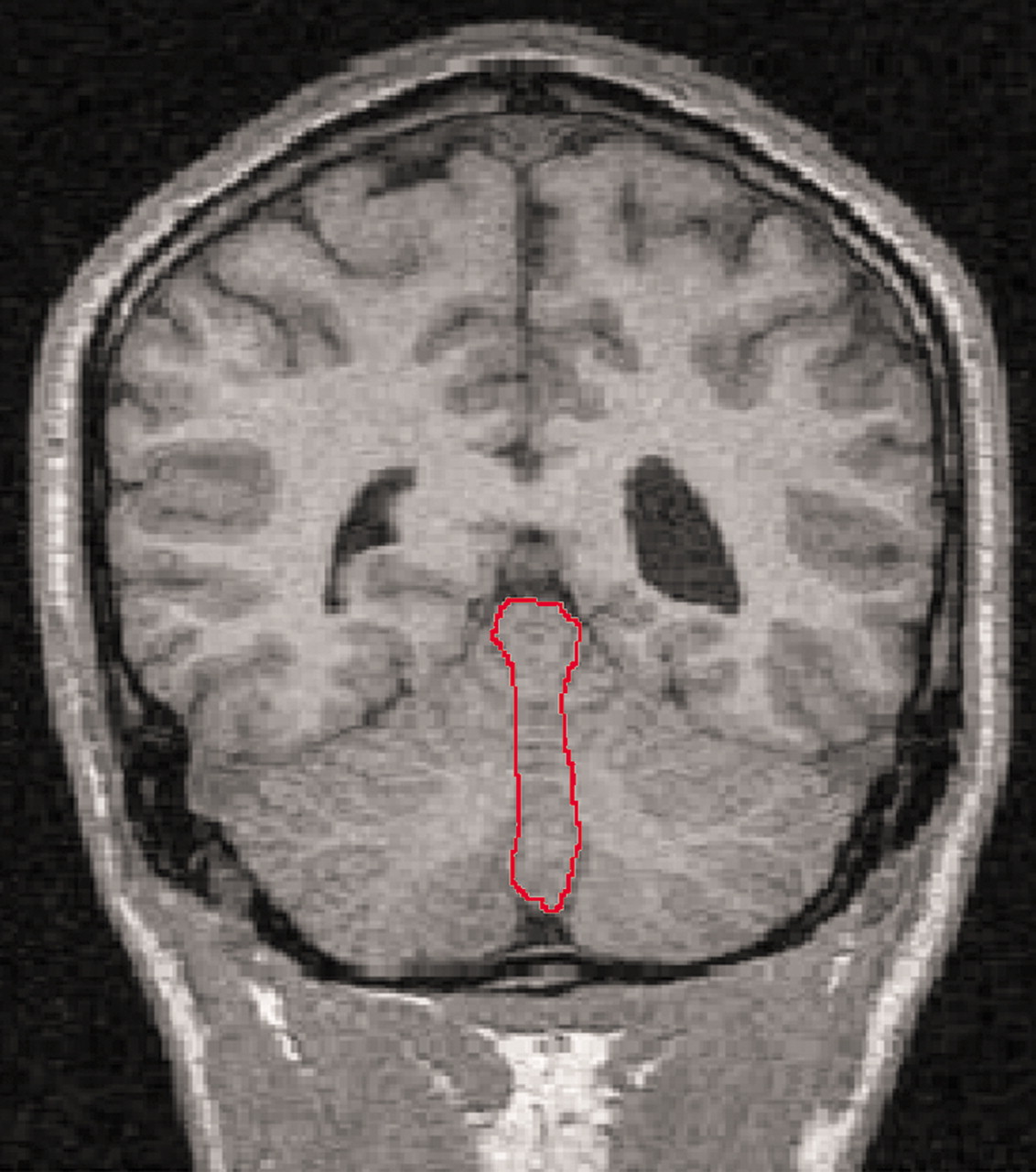
The volumes of the vermal volumes (lobules I–X) were measured manually by outlining these structures where they were visible in the coronal and sagittal slices and checking in three-dimensional orthogonal views (RESCUE) at each slice. The hemispheric tonsils formed the anterior inferior boundary of the vermis. The lateral extent of the vermis was defined by use of a combination of criteria: 1) on the sagittal view, the point where the prepyramidal fissure was no longer visible, 2) the most lateral slice where the corpus medullare retained the typical vermian shape (worm like), and 3) where visible, the paravermian sulcus (
Figure 2). Total cerebellum volumes were measured by manually outlining the cerebellum in all coronal slices in which it was visible, with a perpendicular cut to the direction of the fibers of the cerebellar peduncles visualized on the axial scan. Each cerebellar slice was bisected at the midline for measurements of the right and left cerebellar volumes. The fourth ventricle was measured in all the coronal slices where it was visible. Total brain volumes were measured on the coronal scans, with the cerebellar tonsils as the inferior marker.
The anatomical boundaries of the thalamus were defined on coronal sections with reference to the anatomical atlas of Duvernoy
(21) and the protocol outlined by Portas et al.
(22). The anterior marker was the mammillary bodies, the lateral boundary was the internal capsule, and the dorsal boundary was the lateral ventricle and the third ventricle medially. The posterior marker was the point at which the thalamus merged under the crus of the fornix.
The prefrontal cortex was measured in the coronal plane with the first slice defined at the level of the posterior part of the genu of the corpus callosum and the most anterior slice containing gray matter as the anterior marker.
Reliability Studies
Ten subjects were reexamined by the same investigator (A.J.) and a second rater (S.J.). The intra- and interobserver correlation coefficients
(23) were between 0.86 and 0.90 for vermal volumes, 0.95 for total brain volumes, 0.94 for fourth ventricular volumes, 0.87 for thalamic volumes, and 0.92 for prefrontal cortical volumes.
Statistical Analysis
The mean values, over the two times, for the Positive and Negative Syndrome Scale score, medication dose, and brain volumes and the change in these values (value at time 2 minus value at time 1) were calculated, and these were the variates analyzed. Analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were performed on the mean values and the changes, with (where applicable) diagnosis (schizophrenia, normal), sex (male, female), and (where applicable) their interaction as factors. Various variates were considered as covariates in the ANCOVA, i.e., age, age at time 1, and total brain volume for the regional volumes and change in age, age at time 1, and change in total brain volume for change in regional volumes. Only the analyses indicating significant covariates are reported. Where no covariates were significant, the ANOVA results are reported.
Results
Each diagnostic group (patients with schizophrenia and comparison subjects) had nine males and seven females. The distribution of subjects among the socioeconomic classes defined by the Office of Population Censuses and Surveys
(24) did not differ significantly between groups; the numbers of patients in classes I, II, III, IV, and V were 2, 4, 11, 6, and 1, respectively, and the numbers of comparison subjects were 1, 8, 6, 2, and 0, respectively (χ
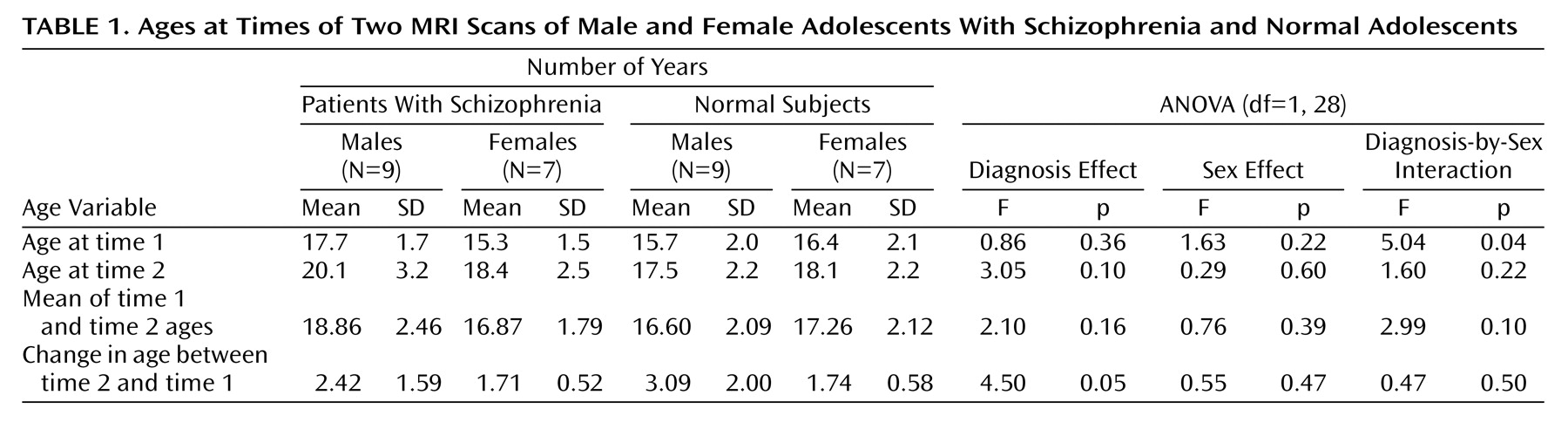
2=5.0, df=4, p=0.28). The subjects’ ages at the two time points are reported in
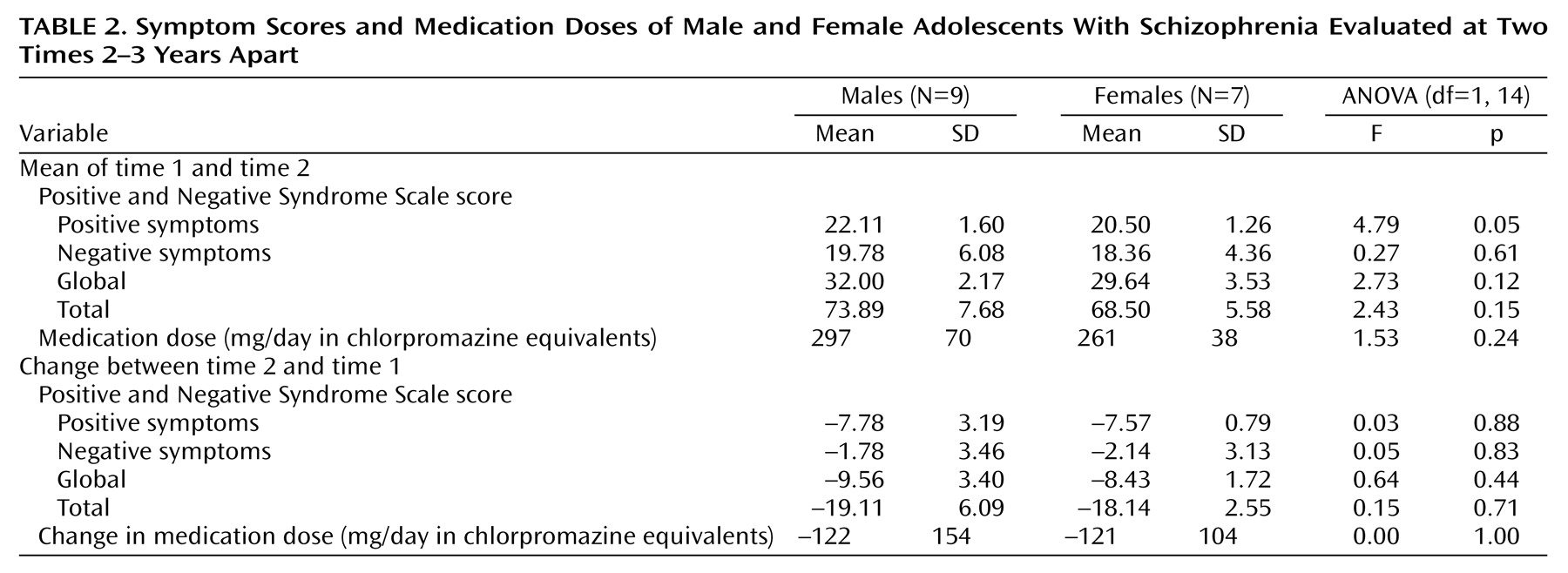
Table 1. It can be seen that the effect of diagnosis on the change in age is just significant. The results of the analyses of symptom scores and medication doses are given in
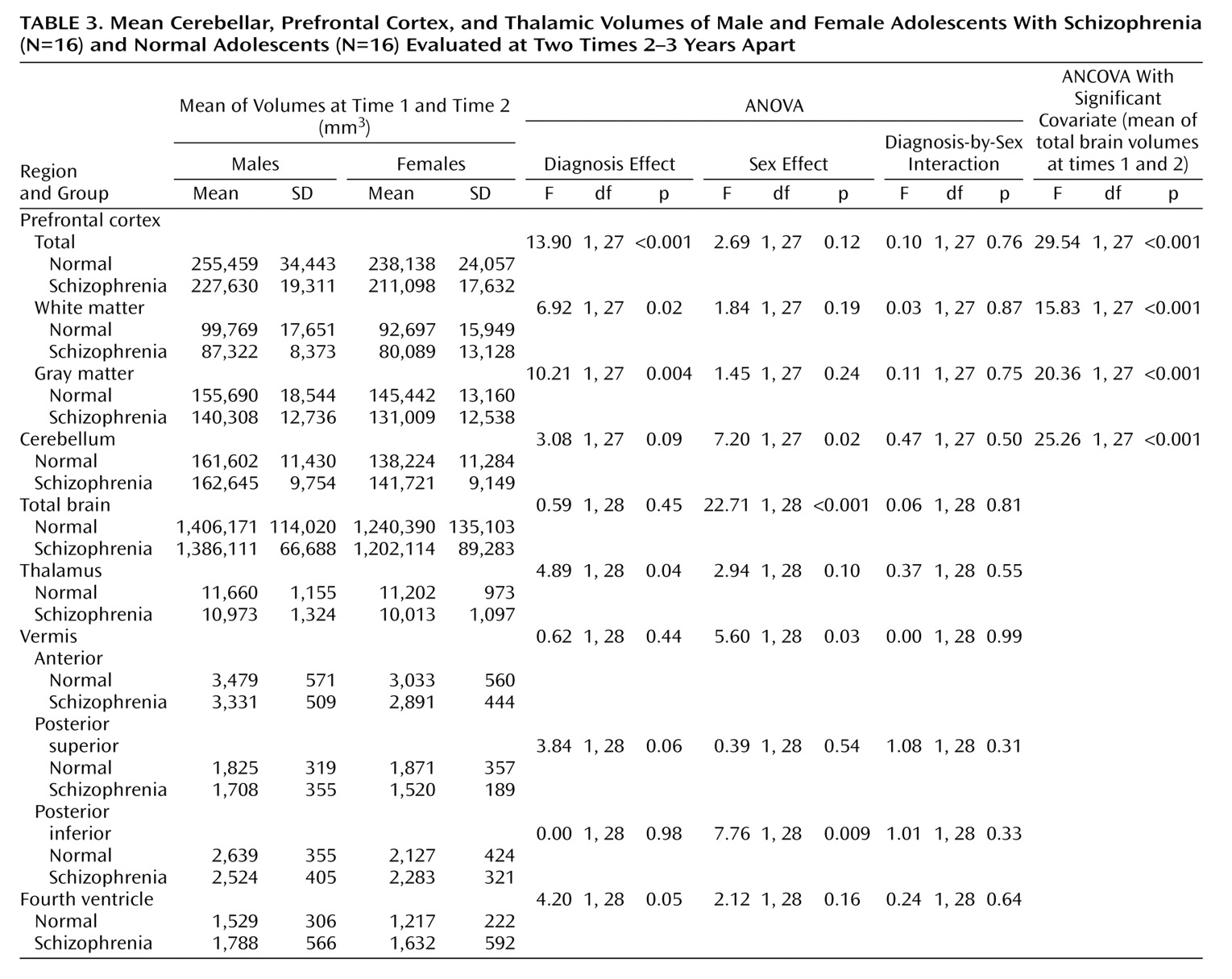
Table 2. The results of the analyses of the mean volumes are given in
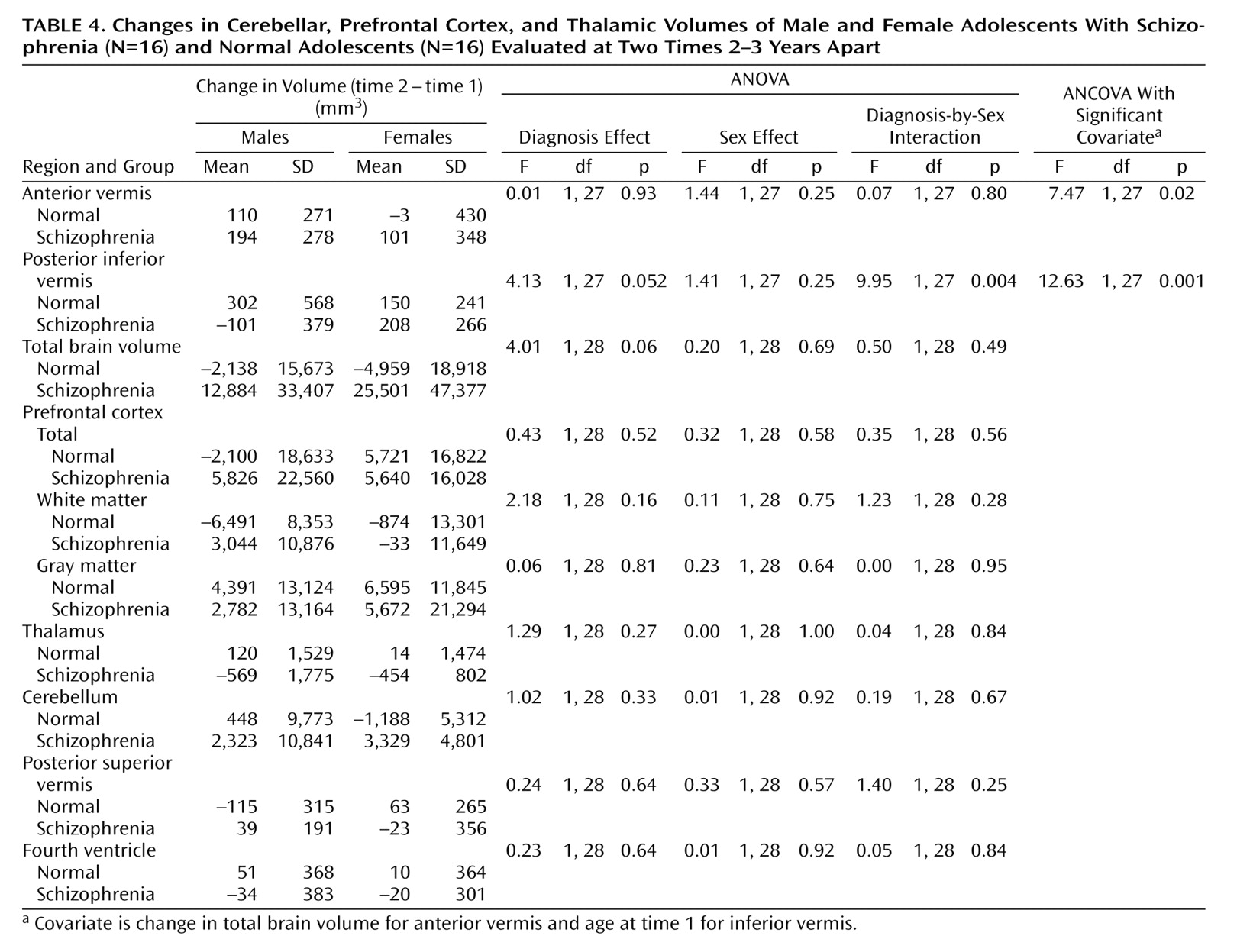
Table 3. There is strong evidence of differences with regard to diagnosis for the prefrontal cortex mean values and moderate evidence for the thalamus means; the schizophrenic patients had lower mean volumes than the normal subjects in both cases. There is some evidence for a difference between groups in the mean volume of the fourth ventricle; in this case, the patients displayed larger volumes than the normal subjects. The results of the analyses of the change in volume are given in
Table 4. No changes were significant with regard to diagnosis, although the differences in the volume of the posterior inferior vermis and the total brain volume were nearly significant and the effect of the diagnosis-by-sex interaction on the volume of the posterior inferior vermis was significant. There was no evidence for significantly altered asymmetry in any of the structures examined.
Discussion
The main findings are strong evidence that patients with schizophrenia have smaller volumes than normal subjects in the prefrontal cortex, moderate evidence for smaller thalamic volumes, some evidence for larger fourth ventricle volumes, and no evidence of progression over time for any of these except the posterior inferior vermis volume among males.
Longitudinal studies of midsagittal thalamic areas in childhood-onset schizophrenia show a progressive reduction over time
(11,
25). In the Edinburgh High Risk Study
(26), involving older subjects, the thalamic volume was found to be low bilaterally in high-risk and schizophrenic subjects, however, the low volume was present premorbidly and was nonprogressive.
A meta-analysis involving 485 schizophrenic subjects (49 adolescents) and 500 comparison subjects showed lower absolute thalamic volumes (effect size=–0.41) and volumes corrected for brain size (effect size=–0.30) in the schizophrenic subjects
(27). The 9.1% lower volume in this study is considerably larger than the 4% reported in a meta-analysis of studies of adults
(28). The difference in prefrontal volumes shown by that meta-analysis (lower in schizophrenic patients) was also less than in our study. These two findings suggest a more severe disease process in early-onset schizophrenia.
In normal adolescence, expansion of the dorsal frontal cortex is associated with lower gray matter density
(29), whereas accelerated gray matter loss (>5%/year) occurs in the frontal regions in childhood-onset schizophrenia
(30). In this study, a 9.6% reduction in prefrontal cortex volume (13.1% for white matter; 7.5% for gray matter), in comparison to healthy subjects, is in line with that reported in childhood-onset schizophrenia
(30) but greater than the 1%–5.5% median reduction reported in a meta-analysis of studies of prefrontal lobe volumes in adults
(31). Hirayasu et al.
(32) reported a significant reduction in left prefrontal gray matter in a follow-up analysis of first-episode patients with schizophrenia (18–29 years of age), while Ho et al.
(33) found a progressive loss of white matter and an increase in frontal lobe CSF over a 3-year period in young schizophrenic adults. The timing of the loss of prefrontal volume is important, with a progressive loss in the first years after the onset of childhood-onset schizophrenia
(30). A progressive reduction in the left inferior frontal volume was reported in a small group of subjects at ultrahigh risk during the transition to psychosis
(34). However, in this post-onset study of patients in late adolescence, there was no reduction over time in the prefrontal cortex volumes.
The importance of the cerebellum in schizophrenia is suggested by its involvement in the higher cortical functions of working memory and language processing, as well as in motor coordination
(35). An early study of childhood-onset schizophrenia showed no differences from comparison subjects in total cerebellar hemisphere volumes or fourth ventricular volumes but did show smaller midsagittal vermal areas and volumes, when corrected for total cerebral volume
(36). However, a recent prospective longitudinal study
(9) revealed a progressive loss of cerebellar volume but no changes in vermal areas or posterior inferior vermal volume. The study was, however, limited to treatment-resistant subjects. There did not seem to be any progressive cerebellar changes in our study, although the loss of the posterior inferior vermal volume approached significance. Again, although not significant, there was an indication of a lower posterior superior vermal volume in the schizophrenic patients than in the comparison subjects. In contrast to the lack of reported fourth ventricular abnormalities in childhood-onset schizophrenia
(36), the larger fourth ventricle in this study appears consistent with this vermal midline difference because of its intimate anatomical relationship. Selectively lower volume of the posterior superior vermis has also been found in men with chronic schizophrenia
(37). The timing of any vermal changes may be important, as evidence
(38) indicates that the cerebellum is one of the latest-maturing of all brain structures, reaching a peak at 19 years, suggesting possible later environmental and/or genetic influences.
There are limitations to this study. First, with only 16 matched subjects at follow-up it lacks certain power to detect differences between groups. However, the effect sizes found were large enough to allow interpretation, with the possible exception of the cerebellar findings. Second, a difficulty in recruiting comparison subjects at the outset of the study meant that the follow-up period differed between groups. As a check, age was used as a covariate in the analysis and generally made no difference to the results. Although limited by the relatively large scan width (3 mm), the findings are nonetheless in line with those based on more automated techniques of deformation-based
(6) and voxel-based
(7) morphometry. Finally, medication effects cannot be discounted, as all the patients were receiving long-term neuroleptics, including clozapine for three patients.
In summary, the evidence from these subjects with adolescent-onset schizophrenia of low volumes of the prefrontal-thalamic structures is consistent with an early-onset, possibly neurodevelopmental basis for a part of the dysconnectivity syndrome. The nonsignificant cerebellar findings add little to the argument for abnormalities of the prefrontal-thalamic-cerebellar circuit because the effect on the cerebellum is likely to be small, too small to be picked up with our small study group. Future studies will need larger groups of subjects. Structural studies are limited and only the first step in the investigation of altered neuronal circuitry. In addition to functional studies, future studies to examine connectivity should include diffusion tensor imaging, which can outline white matter tracts.