Concerns have been raised about the risk of atypical antipsychotic use and cerebrovascular accidents. On the basis of data from four randomized controlled trials (comprising 1,230 elderly dementia patients), more risperidone-treated patients experienced strokes and transient ischemic attacks than did placebo-treated patients (4% versus 2%)
(1). This prompted government regulatory agencies, including the U.S. Food and Drug Administration, to issue warnings of the possible association between risperidone use and cerebrovascular adverse events. Because of the modest number of total subjects enrolled and the small absolute numbers of subjects who experienced cerebrovascular adverse events, it is unclear whether there is a true association. Furthermore, it is unknown if this potential adverse drug reaction is specific to risperidone or is a risk factor for other atypical antipsychotics.
Method
This study utilized a retrospective population-based cohort design that linked administrative health care databases in Ontario, Canada, and included approximately 1.4 million patients over age 65 from April 1, 1997, through March 31, 2002. In Ontario, all patients over age 65 are eligible for coverage of prescription drugs, hospital care, and physician services. The databases were linked anonymously; encrypted individual health card numbers were used to provide information for cohort identification, comorbidity, and clinical endpoints. The study was approved by the Ethics Review Board.
Users of risperidone and olanzapine were compared with those dispensed any typical antipsychotic. Clozapine and quetiapine were not examined because of the relatively small numbers of prescriptions written during the observation period. Users were defined as individuals age 66 and older who were given at least two successive prescriptions and received enough drug for at least 30 days of observation. Duration of exposure was the period of continuous, exclusive use of any of the study drugs starting from the index date. If subjects failed to refill their prescription for the study drug, they were deemed to have discontinued the study drug. Follow-up ended with hospital admission for stroke, exposure to a medication from another study group, discontinuation of the drug, death, or end of the observation period. Hospital admissions for stroke were identified by using ICD-9 codes 430, 431, 434, 435, and 436 to define stroke-related outcomes.
Time-to-event analyses using Cox proportional hazard models were conducted. Unadjusted and adjusted hazard ratios with 95% confidence intervals (CIs) were calculated with typical antipsychotics used as the reference. Covariates chosen for the model included hospitalizations, procedures, and drug utilization hypothesized to be associated with the risk for stroke as well as basic demographic characteristics. The number of prescription drugs dispensed in the year before the index date was included as a measure of overall comorbidity
(2), a measure similar to the Charlson comorbidity index
(3). A pairwise combination of hazard ratios for risperidone and olanzapine were also compared, and assumptions for each exposure variable were assessed for violations. Analyses were performed with SAS for UNIX Version 8.2 (SAS Institute, Cary, N.C.).
Results
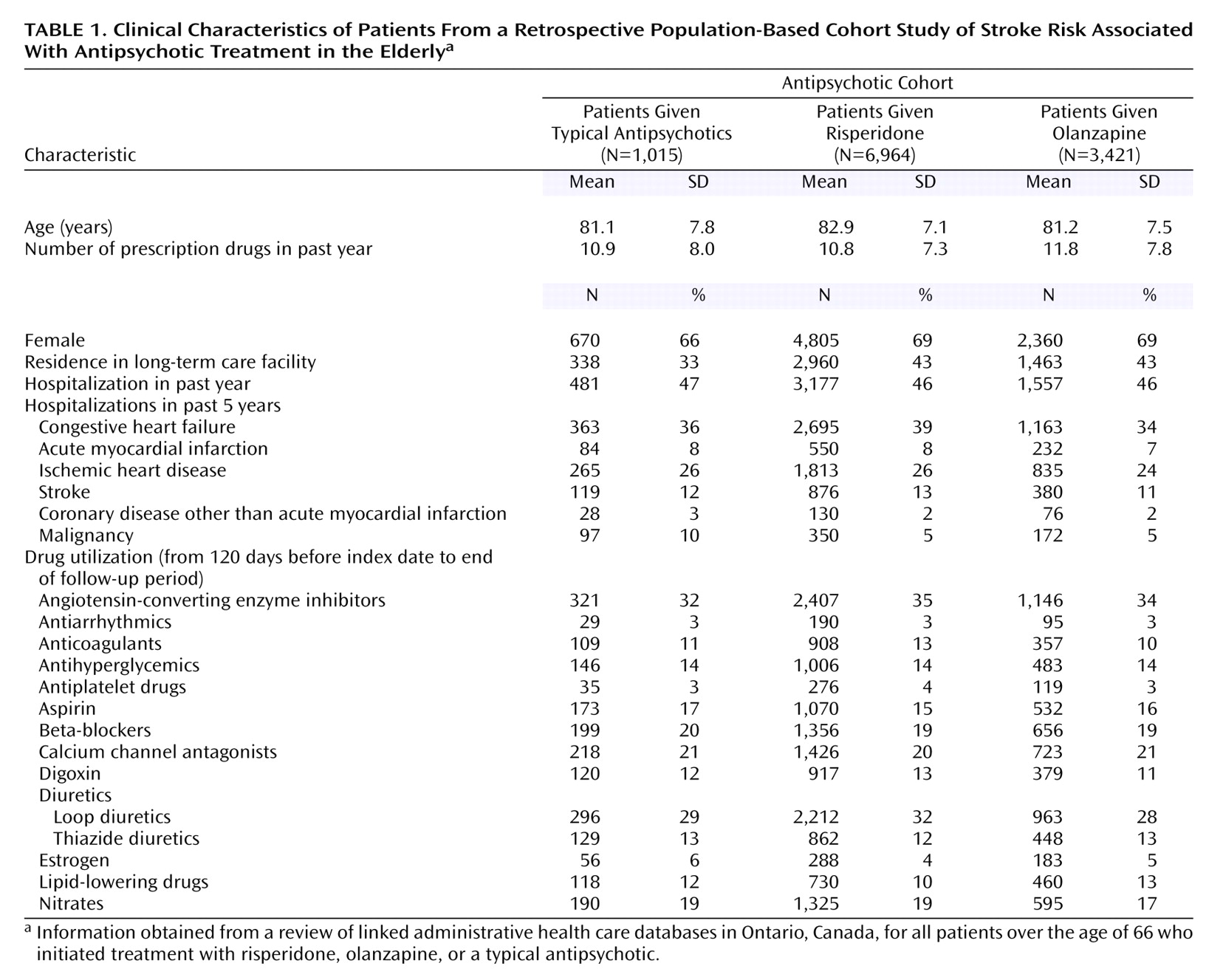
Of the approximately 1.4 million potential subjects, there were 11,400 antipsychotic-naive subjects during the observation period who began a regimen of typical antipsychotics (N=1,015), risperidone (N=6,964), or olanzapine (N=3,421) (
Table 1). Risperidone-treated patients were almost 2 years older on average than patients in the other two cohorts, and fewer typical antipsychotic users were residents of long-term care facilities. The proportion of subjects hospitalized and the number of prescription drugs used in the past year were similar for all the cohorts. These elderly cohorts were medically frail as demonstrated by the number of hospitalizations in the previous 5 years.
During 13,318 person-years of follow-up, there were 92 admissions for stroke (typical antipsychotic users: N=10; risperidone users: N=58; olanzapine users: N=24). The crude stroke rate per 1,000 person-years did not significantly differ among the patients treated with typical antipsychotics (5.7), risperidone (7.8), and olanzapine (5.7). Relative to typical antipsychotic users, model-based estimates adjusted for covariates revealed a risk ratio for stroke of 1.1 (95% CI=0.5–2.3) with olanzapine use and 1.4 (95% CI=0.7–2.8) with risperidone use. Relative to olanzapine, users of risperidone were not at significantly increased risk of stroke-related hospital admission (adjusted risk ratio=1.3, 95% CI=0.8–2.2).
Discussion
This study suggests no statistically significant increase in the risk of stroke associated with the use of risperidone or olanzapine compared with typical antipsychotic use in the elderly. The strength of this study included the ability to follow a large cohort of patients over a lengthy period of exposure and to observe a relatively large number of this rare serious adverse event. While an attempt was made to control for many important confounders, we could not account for other potentially important stroke risk factors such as obesity and smoking. This study also examined all elderly antipsychotic users even though previous studies that suggested an increased risk of cerebrovascular adverse events exclusively involved patients with dementia. On the basis of epidemiological studies, however, it is likely that the vast majority of antipsychotic users in this study were being treated for behaviors associated with dementia as opposed to schizophrenia
(4). Administrative database studies are also limited by their inability to directly measure compliance and appropriateness of use. Furthermore, while we used diagnostic codes for admissions for stroke, other important cerebrovascular events such as transient ischemic attacks and mild strokes not resulting in hospital admission were not captured.
The randomized controlled trials of risperidone use in elderly dementia patients that had suggested an increased risk of cerebrovascular adverse events compared with placebo
(5–
7) included large numbers of individuals with vascular dementia and significant stroke risk factors such as a history of previous stroke, diabetes, hypertension, hyperlipidemia, and atrial fibrillation. There was no matching for these vascular risk factors, and it is unclear how well subjects were treated for these vascular risk factors. In a published study of olanzapine use in elderly dementia patients, there were no reported differences in the rates of cerebrovascular adverse events compared with placebo, although that study did not include patients with vascular dementia, unlike the risperidone studies
(8).
While this study demonstrated no statistically significant increase in the risk of stroke for olanzapine or risperidone in over 11,000 antipsychotic users, the possibility of a type II error to detect small differences in stroke-related outcomes between groups cannot be excluded. Assuming the relative risk of stroke in risperidone-treated patients was 1.4, this would translate into approximately two extra strokes per 1,000 person-years. The fact that we were unable to detect an effect does provide information on the anticipated effect size. Further studies to assess the specific risks of atypical antipsychotic use in elderly dementia patients are required.