The anterior and mediodorsal thalamus are important components of the limbic system, which plays a role in the expression and experience of emotion. Reciprocal connections between the mediodorsal thalamus and the dorsolateral prefrontal, orbitofrontal, cingulate, and insular cortices influence the limbic system by integrating and redistributing emotionally relevant stimuli to the frontal lobes
(1–
3). The anteroventral and anteromedial thalamic nuclei also play a role in this circuit by connecting frontohippocampal structures with the anterior cingulate and entorhinal cortex
(4). Furthermore, the thalamus, particularly the mediodorsal thalamus, is a major target for output from the amygdala
(5), an important temporal lobe limbic structure mediating emotional responses
(6). Together, the mediodorsal and anteroventral/anteromedial nuclei play important roles in connecting subcortical limbic structures to the limbic cortex.
In the present study, we estimated neuronal cell numbers and volumes of mediodorsal and anteroventral/anteromedial thalamic nuclei in subjects from the Stanley Foundation Brain Bank. Four groups were studied: subjects with major depressive disorder, bipolar disorder, and schizophrenia, and an age-matched group of nonpsychiatric comparison subjects.
Results
The four subject groups were matched for several clinical variables. According to ANOVA, the groups did not differ in terms of age, postmortem interval, time in formalin, or brain weight. Chi square analysis indicated that the groups did not differ in terms of gender or laterality (i.e., side of the brain analyzed) (
Table 1).
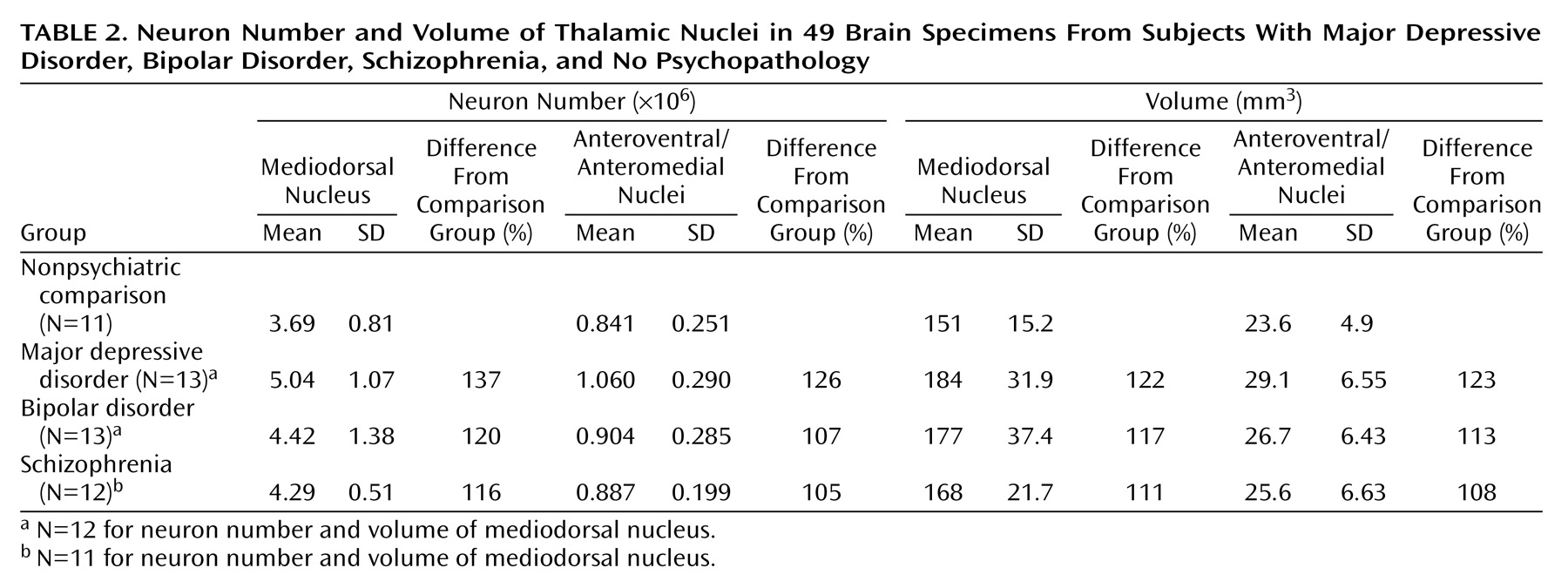
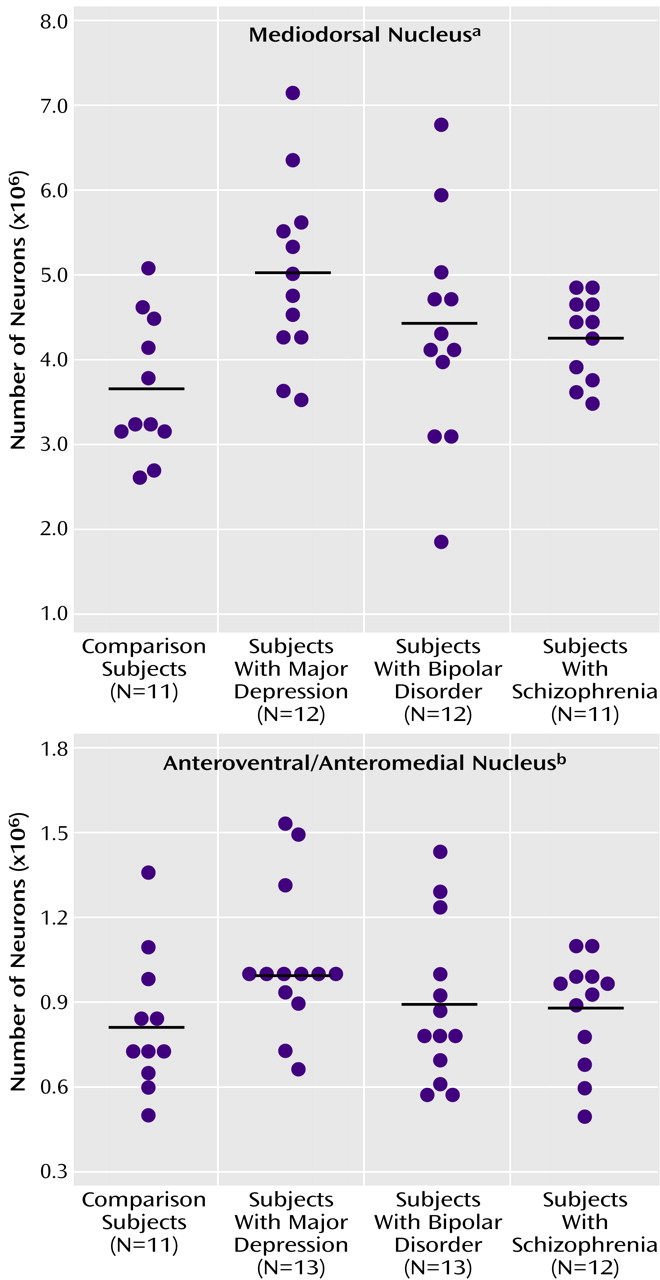
There were more neurons in the mediodorsal and anteroventral/anteromedial nuclei in subjects with major depressive disorder compared with the nonpsychiatric comparison group. Mediodorsal neuron number was significantly different among the four groups of subjects (F=3.42, df=3, 43, p<0.03) (
Table 2). Dunnett’s test indicated that only the major depressive disorder group was significantly different (37% higher) from the nonpsychiatric comparison group. With ANCOVA, the main effect of diagnosis remained significant after accounting for age, postmortem interval, gender, and laterality covariates (F=3.43, df=7, 38, p<0.03), further validating the ANOVA finding of elevated neuron number in the mediodorsal nucleus in major depressive disorder. For anteroventral/anteromedial neuron number data, there was not a significant difference among the four subject groups according to the ANOVA (F=1.60, df=3, 45, p<0.20). However, ANCOVA with covariates of age, postmortem interval, gender, and laterality revealed a significant effect of diagnosis on anteroventral/anteromedial neuron number (F=3.15, df=7, 41, p<0.04). The ANCOVA post hoc test (least squares means Students contrast t test) indicated that only the major depressive disorder group had significantly more anteroventral/anteromedial neurons (26%) than the nonpsychiatric comparison group. The ANCOVA improvement was due in part to correcting the data for laterality and gender effects (right hemisphere structures are larger than left, and females structures are smaller).
Figure 3 illustrates the distribution of mediodorsal and anteroventral/anteromedial cell counts for the four subject groups.
The history of psychiatric drug usage during the subjects’ lifetime was obtained from medical chart review
(14). In order to determine whether antidepressant drug usage influenced the number of neurons in the two thalamic nuclei, we examined mediodorsal thalamic cell number in patients with schizophrenia who were treated (six of 11) versus not treated, and in bipolar cases that were treated (nine of 12) versus not treated with antidepressant medication. Antidepressant treatment did not influence mediodorsal thalamic neuron number in schizophrenia subjects (mean=4.25 million cells [SD=0.55] and 4.32 million cells [SD=0.33], respectively) or bipolar subjects (mean=4.46 [SD=0.86] and 4.23 [SD=0.46]) and did not influence anteroventral/anteromedial thalamic neuron number in the schizophrenia subjects (mean=0.91 million [SD=0.05] and 0.84 million [SD=0.20]) or bipolar subjects (mean=0.88 million [SD=0.32] and 1.0 million [SD=0.32]). Cumulative lifetime antipsychotic drug treatment in fluphenazine equivalents computed from medical records was not significantly correlated with mediodorsal or anteroventral neuron number or volume according to Pearson’s correlation analysis for the schizophrenia (N=11/11), bipolar (N=9/12), or combined (N=20) groups. For the entire cohort, utilization of neuroleptics at any point during the subjects’ lifetime did not have a significant effect on either neuron number or nucleus volume for either thalamic region.
The density of neurons in mediodorsal nuclei did not differ among the four groups of subjects according to ANOVA (nonpsychiatric comparison group: mean=24,654 neurons/mm3 [SD=4880]; major depressive disorder group: mean=27,324 neurons/mm3 [SD=2555]; bipolar group: mean=25,200 neurons/mm3 [SD=6409]; schizophrenia group: mean=25,522 neurons/mm3 [SD=2142]). Similarly, there was not a significant difference in the density of anteroventral/anteromedial neurons among the four groups (nonpsychiatric comparison group: mean=35,408 neurons/mm3 [SD=6232], major depressive disorder group: mean=37,542 neurons/mm3 [SD=8597]; bipolar group: mean=33,619 neurons/mm3 [SD=5165]; schizophrenia group: mean=35,348 neurons/mm3 [SD=4800]).
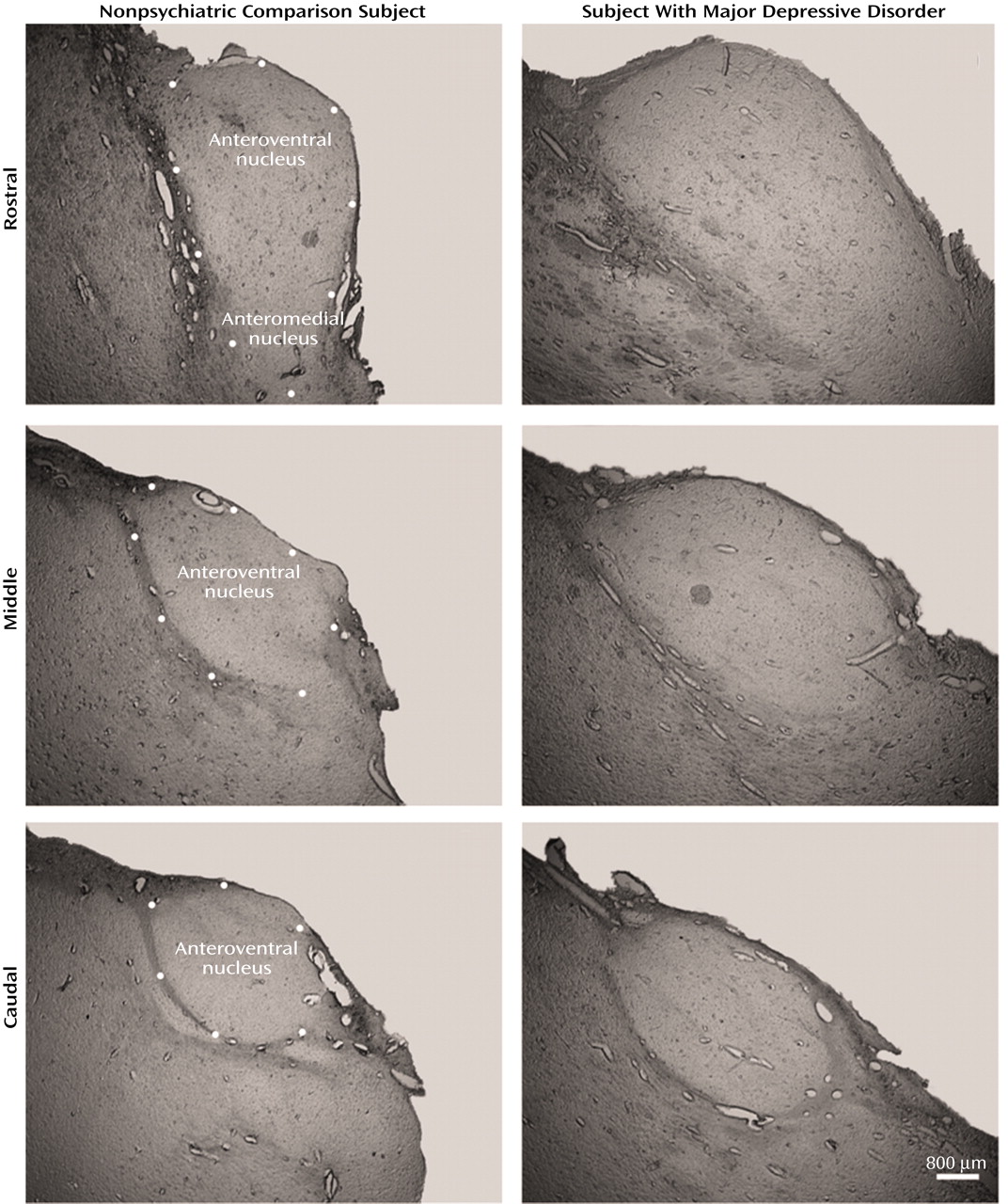
There was a tendency for the volume of the mediodorsal nucleus to be different among the four groups (ANOVA F=2.67, df=3, 43, p<0.06). Post hoc testing (Dunnett’s) indicated a significant difference (22%) between the major depressive disorder and nonpsychiatric comparison groups (p<0.05). However, the ANCOVA analyses with covariates of age, gender, laterality, and postmortem interval did not reveal volume differences among the four groups (F=0.76, df=7, 38, p<0.54). The anteroventral/anteromedial nucleus was not statistically different among the four groups (ANOVA F=1.66, df=3, 45, p<0.19; ANCOVA F=1.95, df=7, 41, p<0.14). We also examined the volume of the total thalamus in major depressive disorder and nonpsychiatric comparison subjects to see if the entire thalamus was larger in major depressive disorder cases. The mean total thalamus volume was 16% greater in the major depressive disorder group versus the comparison subjects, although the result was not significant at the p<0.05 level (comparison group: mean=878 mm3 [SD=105]; major depressive disorder group: mean=1020 mm3 [SD=186]; t=1.78, df=22, p<0.09).
Discussion
In the present study, the mediodorsal and the anteroventral/anteromedial nuclei of the thalamus had significantly more neurons in major depressive disorder cases relative to nonpsychiatric comparison subjects. This is the first study of neuron number in the thalamus of major depressive disorder subjects, and our findings are unique because increases in total neuron number have not previously been observed in psychiatric conditions. Contrary to previous studies, the number of neurons in the mediodorsal and anteroventral/anteromedial nuclei were normal in schizophrenia subjects.
The present data are consistent with other reports of abnormalities in subcortical limbic structures in major depressive disorder
(13). In a structural magnetic resonance imaging study of first-episode depression patients, Frodl et al.
(17) found a bilateral enlargement of the amygdala, and two additional studies report that the amygdala is enlarged in subsets of patients with depressive symptoms
(18,
19). The pituitary gland is also enlarged in major depressive disorder
(20). In the hypothalamus, there are several reports of increased numbers of immunoreactive cells (e.g., arginine vasopressin, vasopressin, corticotropin-releasing hormone) in major depressive disorder
(21–
23). These immunohistochemical studies differ from the present study because they report changes in subsets of immunoreactive cells rather than the total number of neurons. Both the hypothalamus and amygdala are highly interconnected with the mediodorsal and anteroventral/anteromedial thalamus, prefrontal cortex, and cingulate cortex
(5,
24,
25). On the other hand, the caudate nucleus, putamen, and other basal ganglia structures are not increased in volume in major depressive disorder subjects
(26), suggesting that anatomical changes are selective for subcortical structures with limbic connections. Finally, metabolism is increased in subcortical limbic structures in major depressive disorder, including the amygdala and medial thalamus
(27,
28). Increased output from the amygdala to the thalamus, suggested by these previous findings, is particularly interesting since an increase in amygdala metabolism occurs both during episodes of severe major depressive disorder symptoms and after depressive symptoms have resolved
(27). These observations suggest the presence of both structural and functional abnormalities in subcortical limbic structures in major depressive disorder.
All but one of the major depressive disorder subjects in the present study received antidepressant drugs. In animals, treatment with antidepressants increases the rate of neurogenesis, migration, and differentiation in the subgranular zone of the hippocampus and in the marginal ventricular zone
(29). These adult-born neurons can form functional connections
(30). However, the thalamus has not been observed to be a site of antidepressant-induced neurogenesis. Furthermore, approximately 50% of the schizophrenia subjects in the present study had a history of antidepressant treatment, and neither mediodorsal nor anteroventral/anteromedial neuron numbers were elevated in these patients. These data suggest that it is unlikely that antidepressant-induced neurogenesis is responsible for the increase in neuron number in the limbic thalamus in major depressive disorder.
The increase in limbic thalamic neuron numbers in major depressive disorder may be caused by an abnormal neurodevelopmental process. Elevations in mediodorsal and anteroventral/anteromedial neuron numbers could reflect a developmental problem such as an accentuated neuronal birth rate or, alternatively, the survival of excess numbers of neurons. Our data provide no information about which thalamic neuron population (projection neuron or interneuron) is abnormal in major depressive disorder. An elevated number of projection neurons could result in an excess of excitatory glutamatergic output from the thalamus to the cortex in major depressive disorder. Conversely, if numbers of inhibitory interneurons in the limbic thalamus were elevated in major depressive disorder, greater than normal inhibition of thalamic output to the cortex might be present. In humans, many thalamic interneurons are generated in the ganglionic eminence of the telencephalon. Some of these neurons subsequently migrate into the thalamus to become GABAergic interneurons
(31,
32). Further studies are needed to determine whether the “projection” or “interneuron” population is abnormal in the thalamus of major depressive disorder patients in order to better understand the developmental implications and potential pathophysiological effects of thalamic abnormalities in this disorder.
Previous studies have documented abnormalities in the limbic cortex in major depressive disorder, including decreased volume of the medial orbitofrontal cortex
(33), hippocampus
(9,
10), and prefrontal cortex
(7). Also, there is a decreased density of glial cells in the dorsolateral prefrontal cortex
(34) and a decrease in glial cell numbers in subgenual cingulate cortex area 24
(35). These anatomical abnormalities in the cortical target areas of the mediodorsal and anteroventral/anteromedial nuclei may be the result of a decreased cortical input from the thalamus caused by excessive GABA interneuron inhibition of thalamocortical neurotransmission. Decreased input to the cortex could minimize the need for glial support of cortical neurotransmission, affecting cortical glial populations. Alternatively, cortical pathology may be the result of an elevation of excitatory input to the cortex from the thalamus caused by excess numbers of glutamatergic projection neurons. Excess input to the cortex could promote excitotoxicity to cortical cells, including glia. Determination of which populations of thalamic neurons are abnormal in major depressive disorder will be an important goal for future studies.
It has been proposed that a lack of coordinated activity within the limbic system is responsible for some of the symptoms of major depressive disorder
(27,
28,
36). Because the thalamus is a critical connection between the amygdala and the prefrontal cortex, it is well positioned for involvement in major depressive disorder pathophysiology. In particular, GABAergic neurotransmission in the thalamus has a substantial effect on prefrontal cortical metabolism
(37). Excess GABAergic inhibition in the medial thalamus results in decreased frontal cortical metabolism and behavioral deficits such as attention neglect and learning deficits, behaviors consistent with the cognitive symptoms of severe depression. The present data support the hypothesis that thalamic anatomical abnormalities are involved in limbic system dysfunction in major depressive disorder.
In the schizophrenia group, mediodorsal neuron number was normal. These data differ from previous studies in which mediodorsal neuron number was reduced in schizophrenia subjects
(16,
38–40). The number of neurons in the comparison subjects in the present study is similar to our previous study
(16), and similar to estimates reported by others
(39–
42). This observation indicates that the present nonpsychiatric comparison group is not different from other comparison groups with respect to mediodorsal neuron number. In the four previous studies
(16,
38–40) in which mediodorsal cell numbers were reduced in schizophrenia brains, the average age of the subjects was over 60 years. In the present study, the mean age of subjects was 46 years. Because the present data indicate a normal number of limbic thalamic neurons in schizophrenia, additional studies will be needed to investigate the effects of age on thalamic neuron numbers in schizophrenia.
The number of neurons and the volume in the mediodorsal and anteroventral/anteromedial nuclei were normal in subjects with bipolar disorder. Exclusion of four subjects diagnosed with bipolar II disorder, or exclusion of subjects without psychotic features, did not change the overall results. However, it is notable that several bipolar subjects had highly elevated mediodorsal and anteroventral/anteromedial neuron numbers similar to major depressive disorder subjects. Additional studies may identify clinical subgroups of bipolar disorder patients that share some of the anatomical characteristics of major depressive disorder.
The present results should be interpreted with certain limitations in mind. The thalamic tissue blocks were processed in a unique manner consisting of deparaffinization before cryoprotection and sectioning, which may have contributed to a z-axis shrinkage. However, we did not observe significant differences in z-axis thickness between the four subject groups, and our conclusions are unlikely to have been biased by this variable. All anatomical findings, including those of the present study, need to be replicated to verify the uniformity of experimental findings. In particular, volume measurements of the mediodorsal and anteroventral/anteromedial nuclei and of the total thalamus need to be performed with an increased sample size to investigate the tendencies observed in the present study. In the present study, a strength was the inclusion of different neuropsychiatric groups to allow investigation of disease-specific effects. Although the present study represents one of the largest neuropsychiatric cohorts analyzed by rigorous stereological techniques (N=45), the sample size was still not large enough to provide sufficient statistical power to fully characterize potentially important variables such as age, gender, and laterality. The Stanley Foundation brain collection provides a history of drug treatment for all subjects, allowing modest, but still limited, insight into whether the experimental findings of the present study are related to disease or drug treatment. Additional studies will be needed to fully characterize the influences of drug and other therapies. It is thus critical that the present findings be replicated in other cohorts of depressed patients to determine whether the current major depressive disorder population is representative of the disease.
In conclusion, the present study provides evidence for an elevation in neuron number in the limbic thalamus in major depressive disorder, and a normal number of neurons in schizophrenia and bipolar cases. These findings add to the evolving consensus that there is substantial brain pathology in major depressive disorder
(43). The causes and implications of this novel neuroanatomical finding remain to be elucidated.