Several lines of evidence have implicated hypoactivity of
N-methyl-
d-aspartic acid (NMDA) receptors in schizophrenia
(1), and the findings include low brain levels of glutamate carboxypeptidase II (GCPII)
(2). GCPII converts the NMDA antagonist
N-acetyl-aspartyl glutamate to
N-acetyl aspartate and glutamate
(3). GCPII also is found in the intestinal epithelial brush border membrane, where it cleaves glutamate moieties from dietary folyl-polyglutamates to facilitate folate absorption
(4). This dual role for GCPII suggests that both hypofolatemia and clinical symptoms of NMDA hypofunction might reflect low GCPII activity. In addition, folate deficiency could have diverse effects on the neurochemistry of schizophrenia, as folate functions as a single carbon donor in the synthesis of glycine from serine. Folate is also involved in the synthesis of dopamine, norepinephrine, and serotonin through
S-adenosylmethionine methylation pathways
(5) and in the conversion of homocysteine to methionine.
We studied a group of outpatients with schizophrenia to test the hypotheses that folate concentrations would correlate inversely with negative symptoms and that homocysteine concentrations would correlate positively with cognitive impairment and extrapyramidal symptoms.
Method
After giving written informed consent, 100 consecutive outpatients with schizophrenia were enrolled. A research psychiatrist confirmed the diagnosis of schizophrenia and administered the Schedule for the Deficit Syndrome
(6). Patients were excluded if they were taking anticonvulsants or other drugs known to affect folate or homocysteine concentrations (N=3), were actively abusing alcohol (N=5), or had renal insufficiency (N=1). Patients were assessed with the Positive and Negative Syndrome Scale, Scale for the Assessment of Negative Symptoms (SANS), Hamilton Depression Rating Scale, Global Assessment Scale, Simpson-Angus Rating Scale for extrapyramidal side effects, and Abnormal Involuntary Movement Scale (AIMS). A cognitive battery was also administered and included the WAIS-III, Trails A and B, FAS verbal fluency test, Wisconsin Card Sorting Test, Stroop Test, Finger Tapping Test, and California Verbal Learning Test. Phlebotomy was performed, and serum samples (nonfasting) were assayed for folate, total homocysteine, B
12, glycine, and serine concentrations.
To compare folate and homocysteine concentrations in our patients with those of a representative nonpsychiatric sample, we used published mean plasma folate and homocysteine concentrations from a sample of 248 subjects from the sixth examination of the Framingham Offspring Study cohort who were not taking folate supplements
(7).
Serum folate and vitamin B12 concentrations were determined by using cloned enzyme donor immunoassay kits for folate (no boil) and vitamin B12 (no boil) according to the manufacturer’s instructions. The between-day coefficients of variation for the folate and vitamin B12 assays were 6.8% and 7.5%, respectively. Serum homocysteine was measured by a fluorescence polarization immunoassay method with a coefficient of variation of 3.7%–5.2%. Serum serine and glycine concentrations were measured by ion-exchange high-performance liquid chromatography with postcolumn derivatization using ninhydrin and were detected by ultraviolet-visible spectral analysis at 570 nm.
Associations between the clinical rating scale scores and the levels from the serum assays were tested by Pearson’s correlation coefficient. Where smoking status was found to be a significant moderating variable, correlations were also computed separately for the subgroups of smokers and nonsmokers, and analysis of covariance was computed by using the daily number of cigarettes smoked as a covariate. Unpaired t tests were used to test for significant differences in mean scores on the clinical rating scales and the levels from the serum assays between the subjects with and without the deficit syndrome. To protect against biased p values due to unequal group sizes, the nonparametric Mann-Whitney U test was also used. All analyses were two-sided, and alpha was set at <0.05.
Results
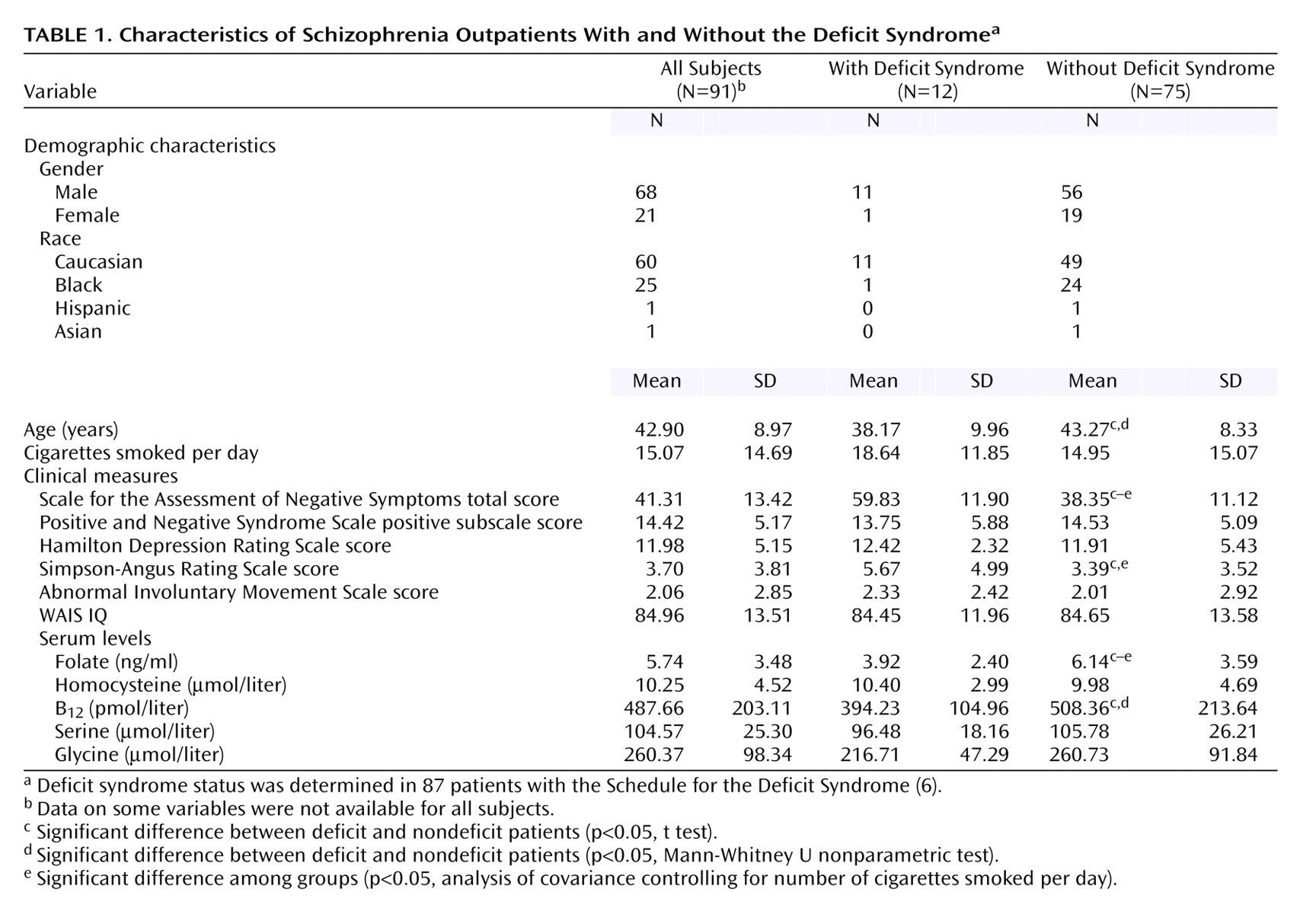
Results of the clinical ratings and serum assays were recorded for 91 subjects, who constitute the study group for statistical analyses (
Table 1). Four subjects were not classified in terms of the deficit syndrome because of insufficient longitudinal clinical data. The mean folate level of 5.74 ng/ml in our total group was significantly lower than the population value of 10.00 ng/ml found in the Framingham Offspring Study (t=11.63, df=31, p<0.001). Smokers had a mean folate level of 4.87 ng/ml (SD=2.22), which was significantly lower than the level of the nonsmokers, who had a mean level of 7.21 ng/ml (SD=4.60) (t=3.25, df=89, p<0.01). The folate levels of both the smokers (t=17.37, df=56, p<0.001) and nonsmokers (t=3.53, df=33, p=0.001) were significantly lower than the level from the Framingham Offspring Study. The folate concentration did not differ significantly between men and women (mean=6.09 ng/ml, SD=3.80, versus mean=4.99 ng/ml, SD=1.97) (p=0.20), did not differ between patients taking atypical and typical antipsychotic medications (mean=5.54, SD=3.44, versus mean=5.95, SD=3.55) (p=0.57), and did not correlate with age. The homocysteine concentration did differ between men and women (mean=10.69 μmol/liter, SD=4.92, versus mean=8.38, SD=1.85) (t=2.09, df=87, p=0.04) but did not correlate with age or number of cigarettes smoked daily and did not differ significantly from the value for the Framingham Offspring Study.
The folate concentration significantly negatively correlated with the SANS total score (r=–0.31, N=91, p<0.01) and was significantly lower in the patients with the deficit syndrome than in those without the deficit syndrome (
Table 1). The correlation between SANS total score and folate concentration was significant in nonsmokers (r=–0.40, N=34, p=0.03) but not in smokers (r=–0.05). Folate and homocysteine levels were not significantly correlated with any of the cognitive measures (for all, r<0.20). The homocysteine serum concentration correlated significantly with the Simpson-Angus Rating Scale total score (r=0.29, N=91, p<0.01) but not with the AIMS score.
The folate concentration did not correlate with the glycine or serine concentration nor with the ratio of glycine to serine (p>0.10). The SANS total score significantly negatively correlated with the glycine (r=–0.29, N=87, p=0.001) and serine (r=–0.23, N=87, p=0.03) serum concentrations. A multiple regression using gender, cigarettes per day, and folate, glycine, serine, B12, and homocysteine concentrations as independent variables revealed that only the folate and glycine concentrations remained significant predictors of the SANS total score (p<0.05). Results of additional analyses are available by request.
Discussion
Serum folate concentrations in this group of schizophrenia outpatients significantly correlated with the severity of negative symptoms, an association that is consistent with hypothesized links to GCPII activity in the gut and brain. Similar to previous results
(8–
11), folate concentrations were lower than those of subjects in the Framingham Offspring Study, a geographically proximate sample who were not taking folate supplements and who were sampled after implementation of folate enrichment of foods in 1997
(7). Whereas hypofolatemia (<3 ng/ml) was found in only 1.7% of the Framingham sample, 16.4% of our study group met the criterion for hypofolatemia.
Differences in folate concentrations between our subjects and the Framingham sample may partly reflect higher smoking rates among schizophrenia patients than among the general population, but the difference remained significant even when nonsmoking patients were compared to the Framingham sample. In fact, the apparent lowering of folate concentrations by cigarette smoking may have obscured the inverse relationship between folate and negative symptoms, since the correlation was significant only among nonsmokers.
Factors other than GCPII activity could also account for our findings. Low folate levels in our subjects may reflect reduced dietary intake, as was reported among schizophrenia inpatients
(9). If patients with negative symptoms ingest less folate because of idiosyncratic dietary patterns, low folate levels could be a result, rather than a cause, of negative symptoms. The lack of a relationship between folate concentration and the ratio of glycine to serine concentrations suggests that folate’s link to negative symptoms is unlikely to be mediated by its involvement in the conversion of serine to glycine.
Homocysteine concentrations did not correlate with cognitive deficits despite the reported association between hyperhomocysteinemia and dementia
(11). The correlation between homocysteine concentration and the severity of extrapyramidal symptoms is of interest, given findings linking homocysteine to dopamine-mediated toxicity and the pathogenesis of Parkinson’s disease
(11,
12). We did not replicate the finding of Levine and colleagues
(13) of high homocysteine concentrations (mean=16.4 μM) in a group of Israeli schizophrenia patients, possibly because of folate supplementation of enriched grain products in the United States.
In summary, serum folate concentration significantly inversely correlated with severity of negative symptoms, a relationship that was significant only in nonsmokers. Glycine and serine serum concentrations also correlated with negative symptom severity; after controlling for multiple factors, we found glycine and folate to predict negative symptoms independently. Dietary intake, GCPII polymorphism status, and cigarette smoking might all play roles in determining serum folate concentration, although cigarette smoking status was not related to negative symptoms in our study group. Low folate may also contribute to negative symptoms through effects on the synthesis of glycine or monoamine neurotransmitters. Future studies to examine factors contributing to low folate concentrations in schizophrenia are needed, as are controlled trials of folate augmentation.