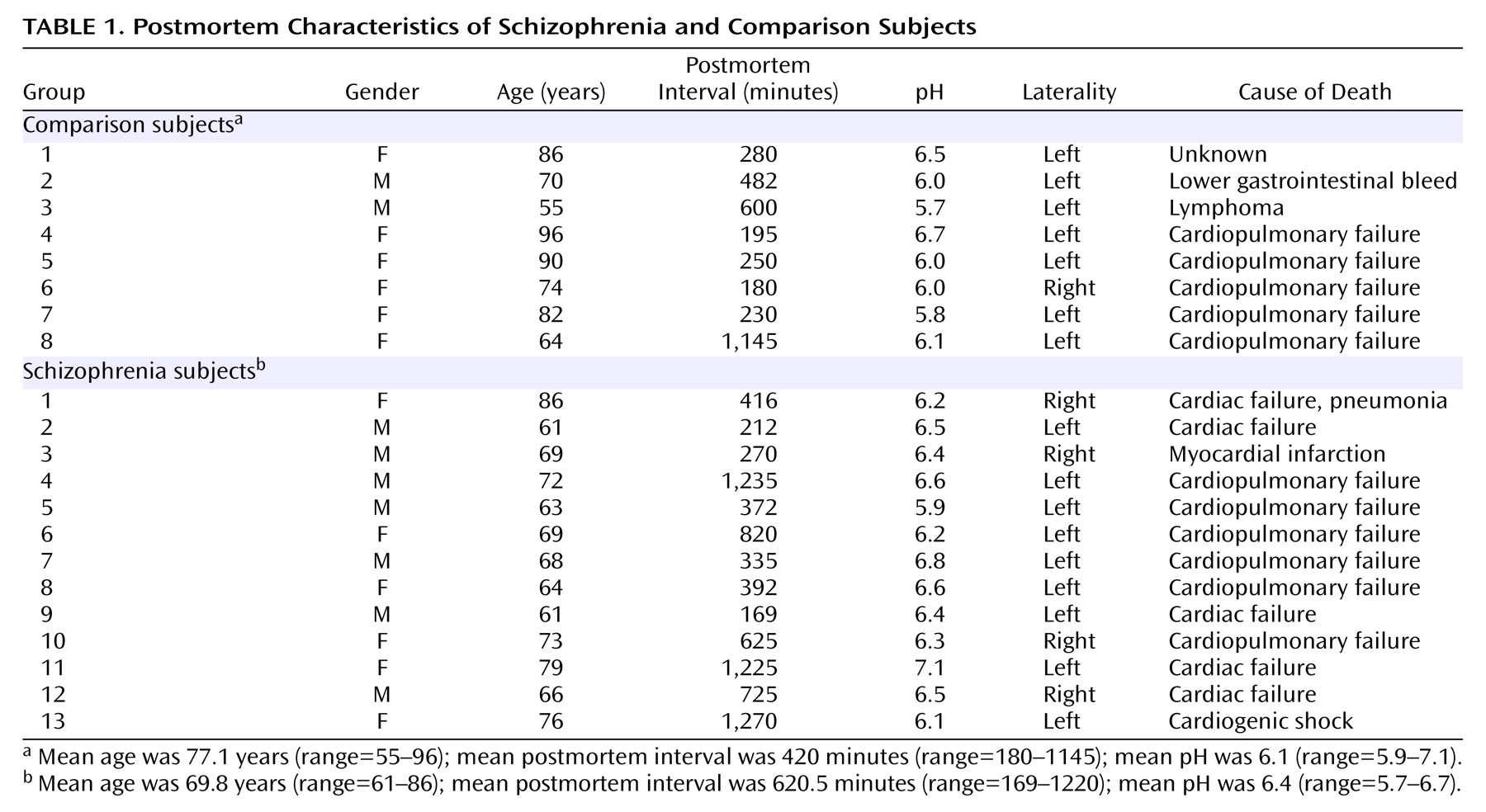
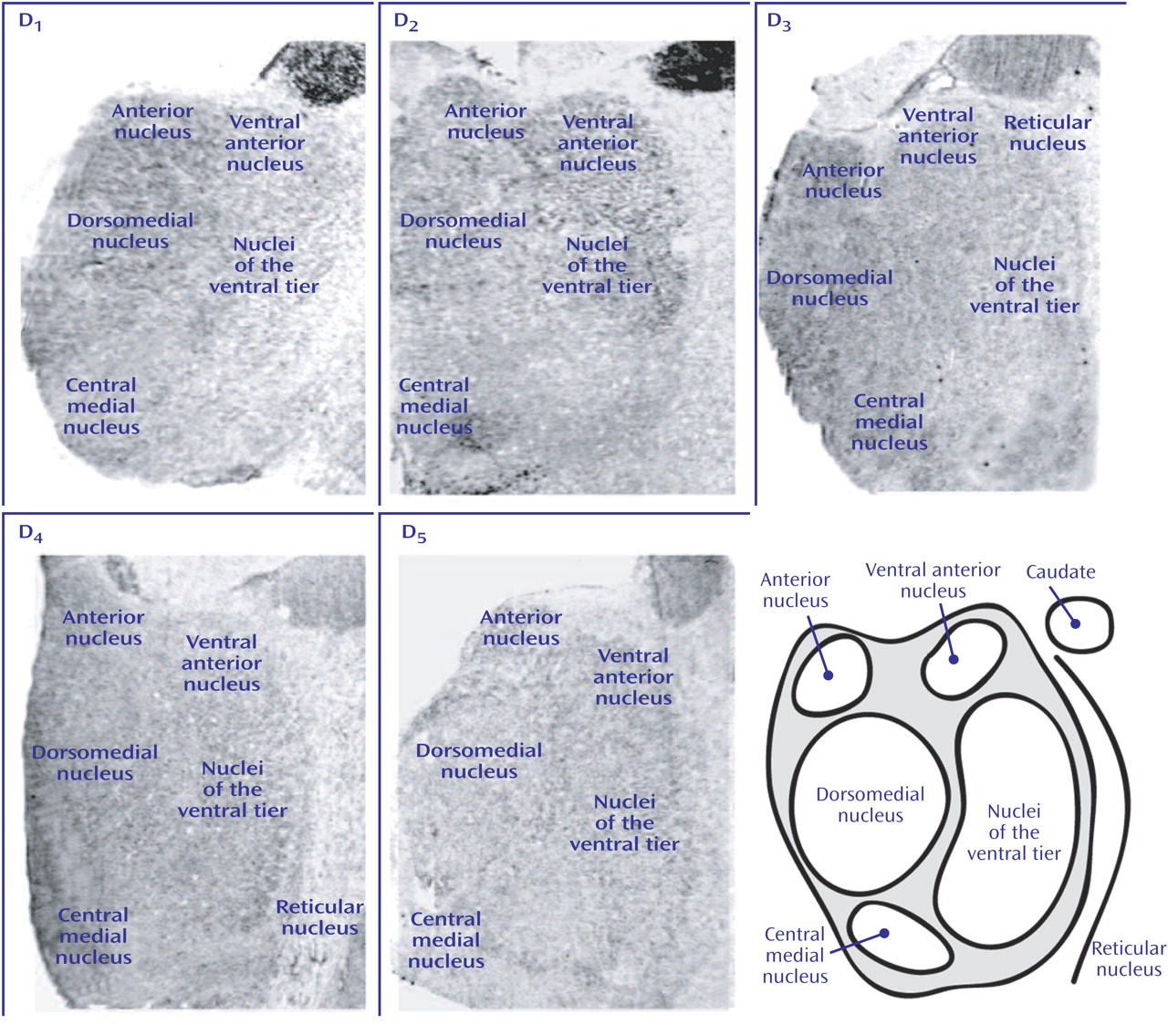
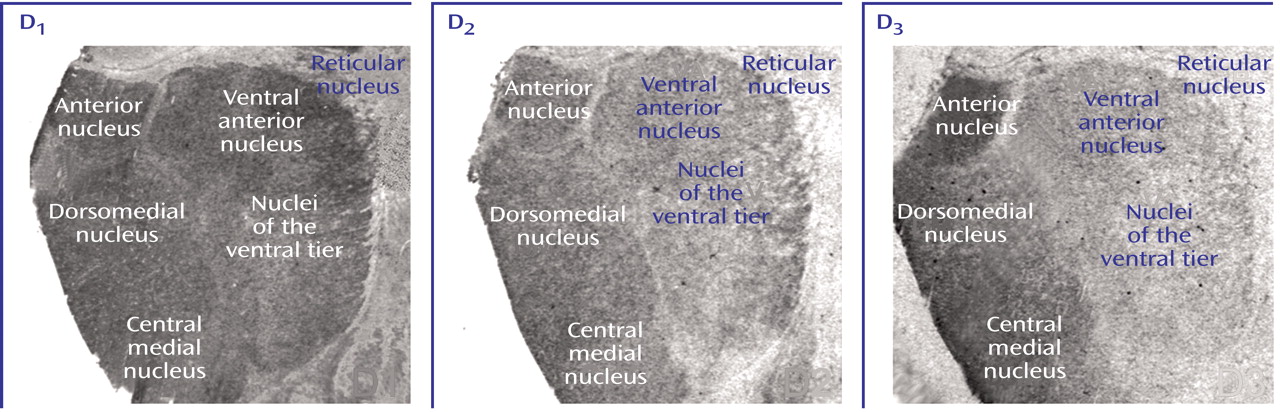
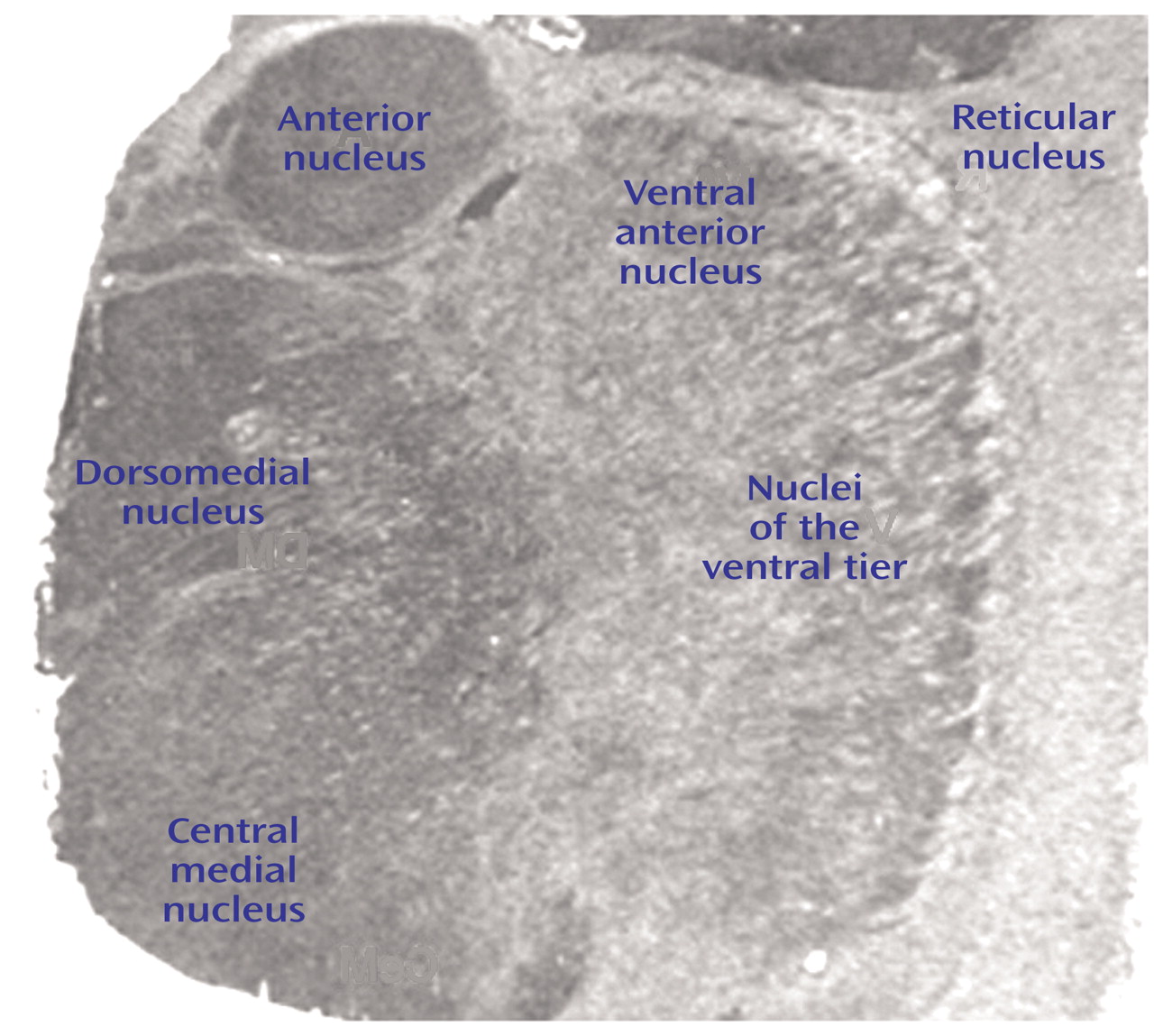
D
1-like receptors primarily couple to G
s proteins to stimulate cAMP formation
(5), but this also leads to increased levels of inositol phosphates
(6) and intracellular calcium
(7). One of the D
1 receptor-linked cAMP signaling pathways involves cAMP-dependent protein kinase, which phosphorylates DARPP-32 (dopamine- and cAMP-regulated phosphoprotein), a potent inhibitor of protein phosphatase-1
(8). The DARPP-32/protein phosphatase-1 cascade controls the phosphorylation states of myriad downstream effectors and therefore exerts a powerful effect on neuronal function
(8). Another recently identified transmembrane protein, calcyon, interacts with the D
1 receptor and mediates intracellular calcium mobilization, permitting “crosstalk” between expressed D
1 receptors and G
q/11-protein-coupled receptors such as metabotropic glutamate receptors and certain muscarinic cholinergic receptors
(9).
D
2-like receptors couple to either G
i or G
o proteins to inhibit adenylyl cyclase and decrease cAMP production
(4), modulate intracellular calcium levels and arachidonic acid release, and increase outward potassium currents
(10). Yeast two-hybrid techniques have shown that the D
2 receptor binds to the protein spinophilin, which is enriched in dendritic spines and interacts with actin and protein phosphatase-1
(11). This D
2/spinophilin interaction may be important for building an intracellular signaling complex that modulates interactions between D
2 and neighboring receptors, especially the AMPA subtype of glutamate receptor, as well as their downstream effectors
(11,
12). While most studies of dopaminergic abnormalities in schizophrenia have focused on the dopamine receptors, there has been recent interest in potential abnormalities of some of the intracellular proteins associated with the transduction of dopamine-mediated signaling
(13,
14).
Superimposed on dopaminergic dysregulation in schizophrenia is the neuroanatomical circuitry that underlies this illness. While earlier hypotheses viewed schizophrenia as a disorder involving discrete brain regions, recent models often conceptualize schizophrenia as a disorder of the functional integrity of distributed circuitry
(15,
16). The predominant neuroanatomical structures hypothesized to be abnormal in schizophrenia include the prefrontal cortex and other cortical and subcortical limbic regions. A critical neuroanatomical structure that links all these areas is the thalamus. The thalamus consists of discrete, topographically organized nuclei with reciprocal projections to and from limbic, sensory, and motor regions of the cortex. Further, much information reaching the cortex from subcortical areas converges on the thalamus before being distributed to cortical regions. While earlier views tended to relegate thalamic function to a simple relay station, thalamic nuclei play a pivotal role in gating and processing information sent to the cerebral cortex. Fibers from brain regions implicated in schizophrenia, including the prefrontal and cingulate cortices, hippocampus, and amygdala, terminate in several nuclei of the dorsal thalamus (e.g., dorsomedial, anterior, lateral dorsal, and central medial nuclei
[17]). The fundamental plan of thalamic circuitry is straightforward: afferent and efferent neurotransmission of all dorsal thalamic nuclei is mediated by glutamate and, in turn, regulated by inhibitory GABAergic effects exerted by neurons of the reticular nucleus and by intrinsic interneurons of the thalamus. Monoaminergic and cholinergic afferents modulate neurotransmission in both the dorsal thalamus and reticular nucleus
(17).
Discussion
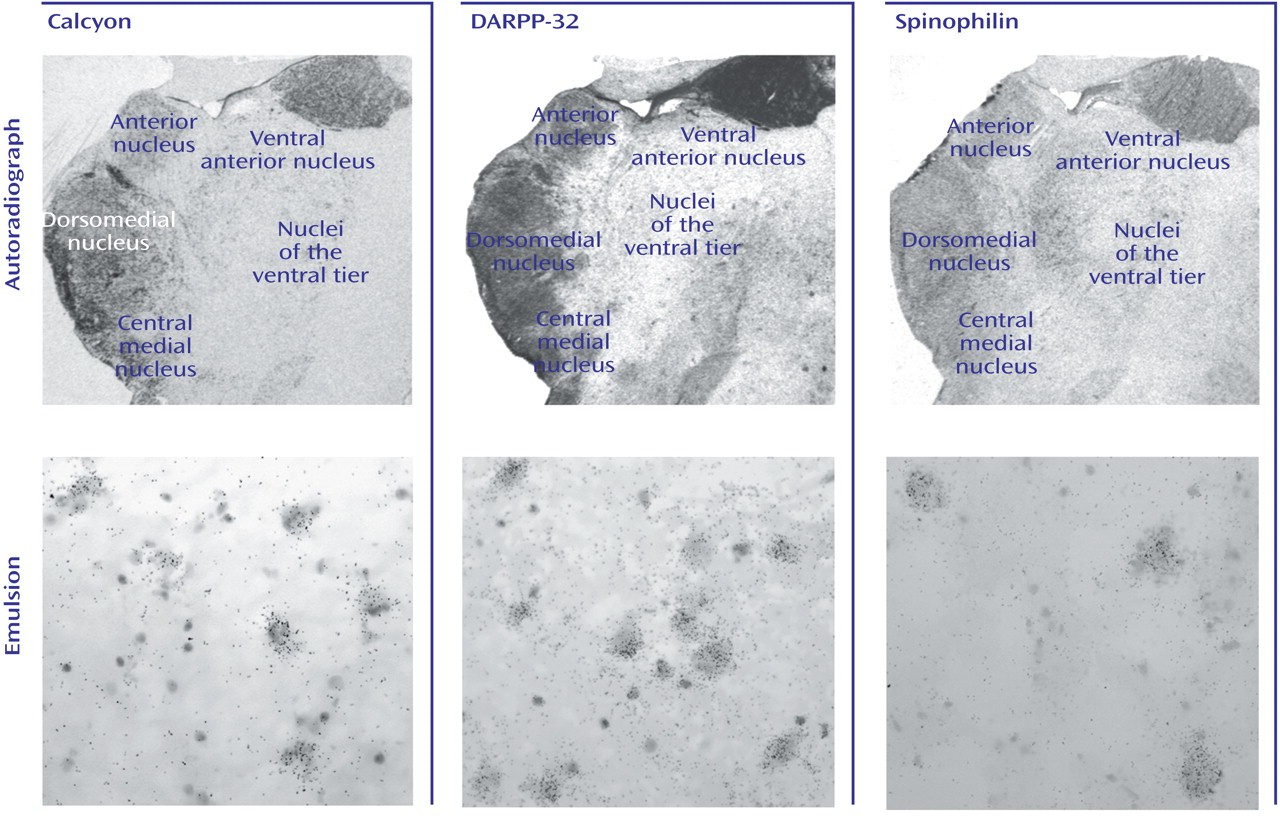
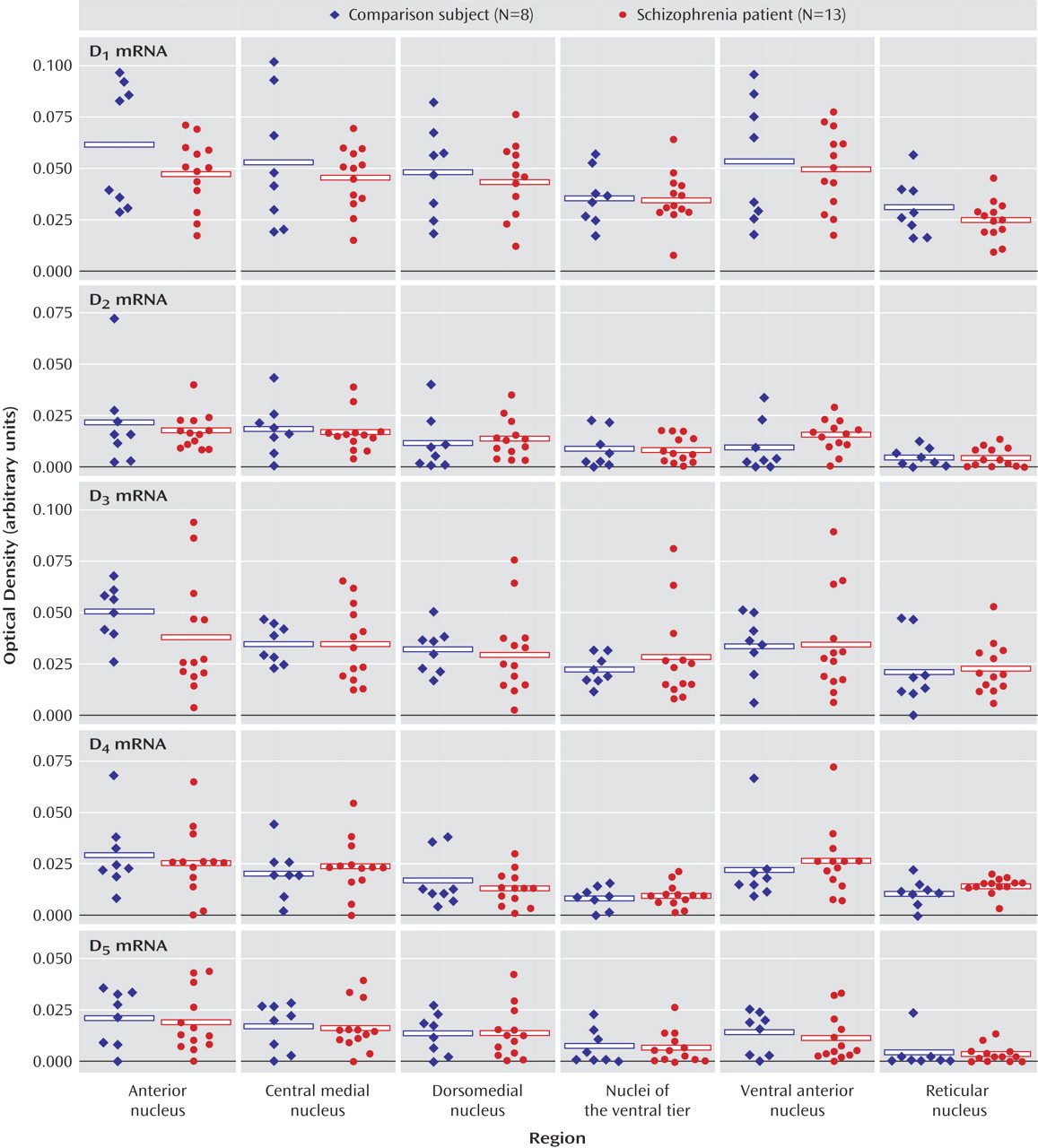
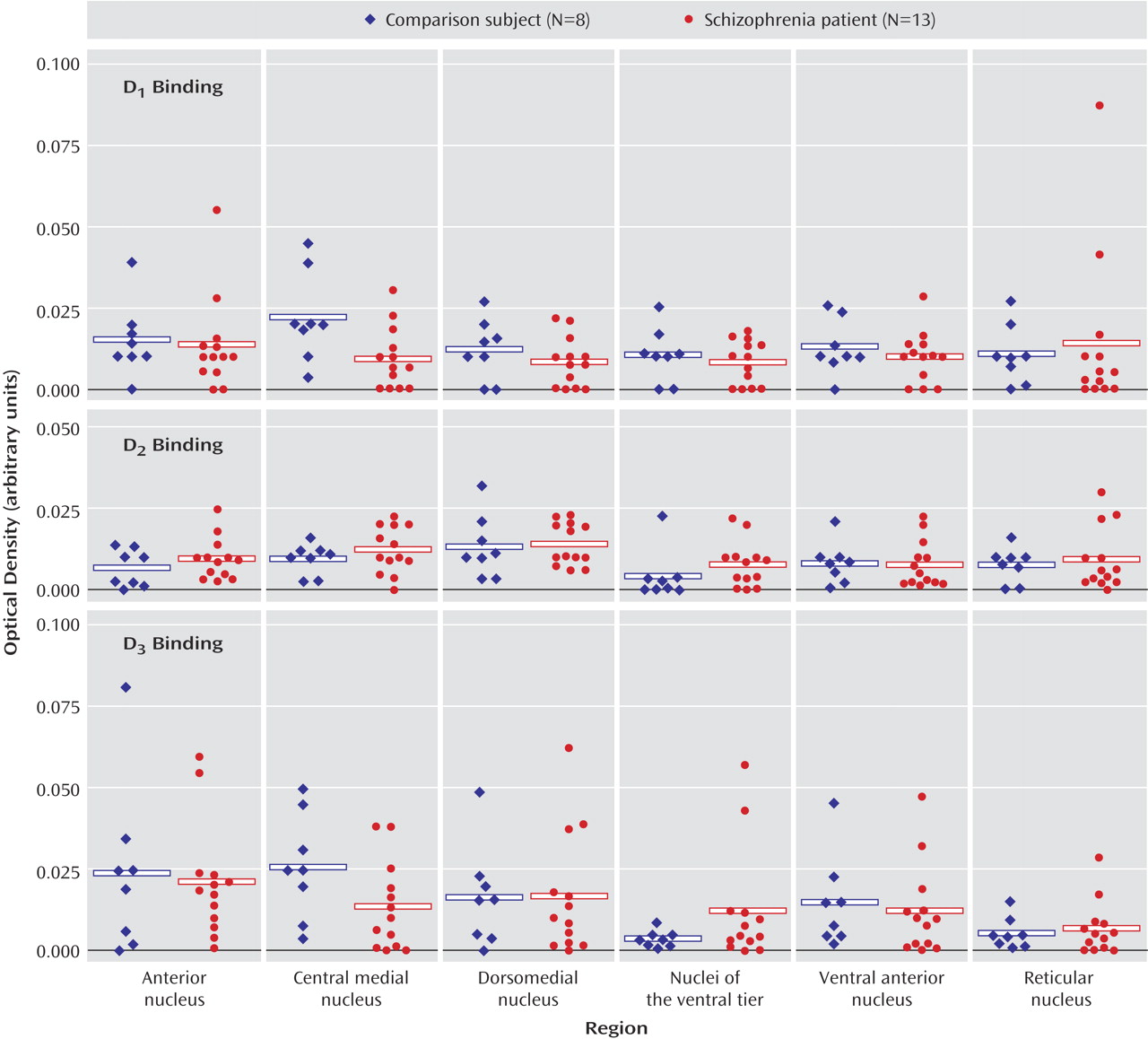
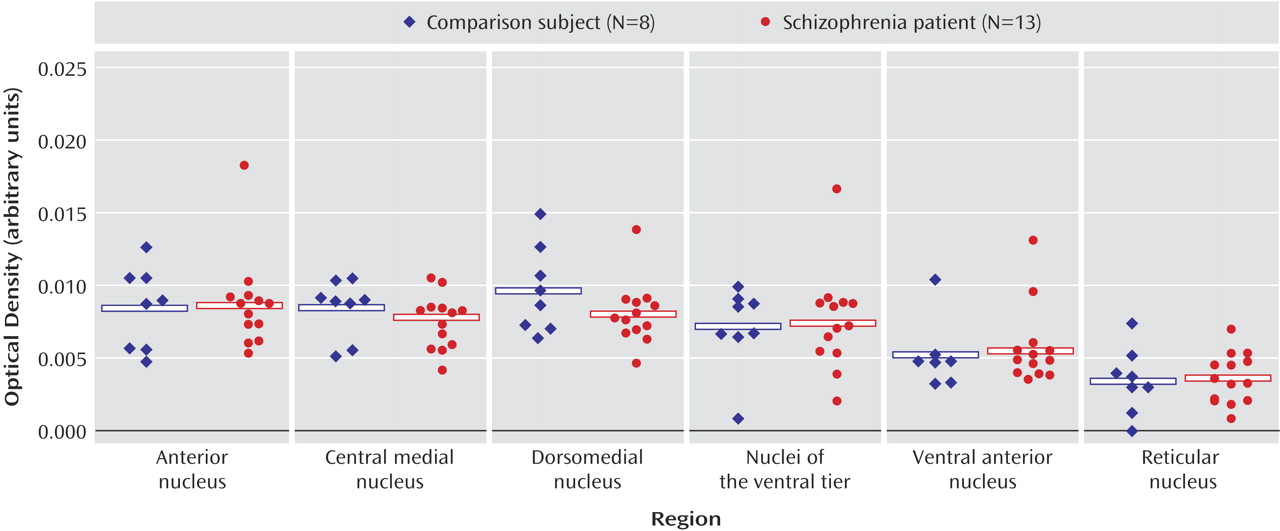
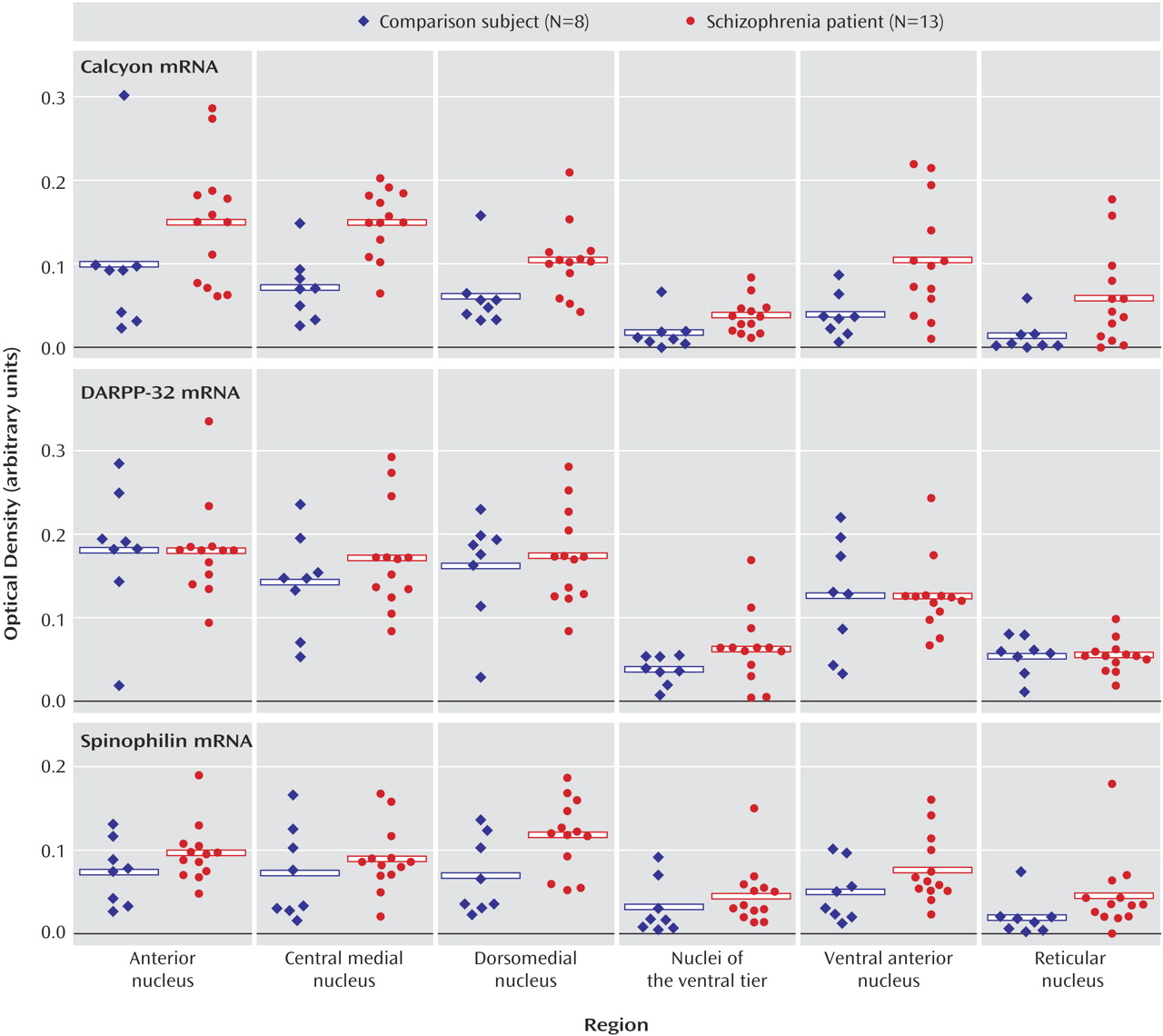
These data indicate that dopaminergic neurotransmission in the thalamus is potentially impaired in schizophrenia. It is interesting that the expression of dopamine receptors is spared in the thalamus in schizophrenia, as is presynaptic innervation as determined by binding to the vesicular monamine transporter. On the other hand, expression of both calcyon and spinophilin, intracellular molecules associated with the integration of dopaminergic neurotransmission with signaling from other neurotransmitter systems in the dendritic spine, are grossly abnormal. These data suggest that dopamine signaling is altered in the thalamus in schizophrenia, but at the level of intracellular signal integration.
Relatively little is known about the normal neurochemical anatomy of the dopamine system in the thalamus. Studies on rodents have revealed only sparse dopaminergic innervation to a few thalamic nuclei, including the dorsomedial nucleus
(41–
44). This is typically associated with low levels of dopamine receptors
(45,
46) with several exceptions: increased densities of D
2 receptors have been noted in the anterior and lateral dorsal nuclei, and high densities of D
4 receptors were reported in the reticular nucleus
(47–
49). Studies on rodents and primates have also typically revealed low levels of transcripts encoding dopamine receptor subtypes in thalamus, although these earlier studies tend to have focused on other structures and only note thalamic expression in passing
(39,
50–53). One study focused on human brain D
2 and D
3 dopamine receptor expression noted specific nuclear distributions in the thalamus
(54). Our dopamine receptor data are consistent with most of these earlier reports: transcripts for all five of the dopamine receptors were detected in human thalamus but at very low levels of expression. Similarly, D
1, D
2, and D
3 receptor binding were all detected in the thalamus. It is interesting that both D
2 and D
3 binding were enriched in more medial, limbic-associated nuclei, which is consistent with earlier work showing relatively increased expression of D
2 and D
3 transcripts and binding in the anterior and dorsomedial nuclei of the human thalamus
(54). Furthermore, our studies of VMAT2 binding and the three intracellular protein transcripts revealed a similar pattern of expression, with enriched levels expressed in medial and anterior nuclei. Taken together, these data support a role for dopaminergic modulation of human thalamic function that is more extensive in limbic-related nuclei.
While a large body of work has examined dopaminergic abnormalities in the striatum and prefrontal cortex in schizophrenia, relatively little attention has been focused on alterations of molecules associated with this neurotransmitter in the thalamus. A few studies have suggested that dopamine levels are elevated in the thalamus in schizophrenia. Using high-performance liquid chromatography, one group found a substantial elevation of dopamine but not norepinephrine levels in the thalamus in schizophrenia
(28,
55). These authors presented detailed maps for the thalami from selected comparison and schizophrenia patients, highlighting “dopamine hot spots” where dopamine/norepinephrine ratios were especially high
(28). The thalami of comparison subjects in this work contained very little endogenous dopamine, but there was significant enhancement of dopamine/norepinephrine ratios in many regions of the thalami in schizophrenia subjects. The pattern of “dopamine hot spots” widely varied between the thalami from each of the patients, however, making these data difficult to interpret. Using a similar assay, this group found significant differences in thalamic dopamine levels within a group of rats that all were handled, housed, etc. under identical conditions, suggesting that technical matters may have confounded this experiment
(56).
This group speculated that the observed increase in thalamic dopamine content might be due to increased dopaminergic innervation of the thalamus in schizophrenia
(28). We have examined afferent monoaminergic innervation to the thalamus by measuring binding to VMAT2 as measured by MTBZ. VMAT2 is the transporter responsible for neuronal translocation of neurotransmitter monoamines from cytoplasm to synaptic vesicles and is expressed in neurons employing the neurotransmitter dopamine, norepinephrine, serotonin, or histamine
(57–
59). Unlike alternative markers of synaptic terminals, the expressed concentration of VMAT2 appears to reflect the density and integrity of presynaptic innervation and is relatively unaffected by counterregulatory and compensatory changes accompanying drug treatment. The vast majority of data supporting this interpretation are derived from study of the nigrostriatal dopaminergic projection. Experimental animals treated with a range of drugs affecting dopaminergic neurotransmission (including D
2 dopamine receptor agonists, D
2 antagonists, dopamine reuptake inhibitors, and dopamine metabolism inhibitors) do not significantly alter the expressed levels of VMAT2 in the striatum
(59,
60) or in the cerebral cortex
(61). Conversely, interventions associated with the irreversible lesions of nigrostriatal neurons or terminals
(40,
62) result in reduced striatal VMAT2 binding levels, reflecting lesion severity
(40). We interpret our present MTBZ data as a reflection of the distribution and density of presynaptic monoaminergic terminals in the thalamus. Given the striking parallels among VMAT2, D
2, and D
3 binding sites and calcyon and DARPP-32 transcripts, we suspect that VMAT2 is to a large extent reflecting dopaminergic innervation in the thalamus. Our data suggest that there is no change in monoaminergic innervation to the thalamus in schizophrenia.
Similarly, there were no changes found for either dopamine receptor transcripts or binding sites in schizophrenia subjects. On the other hand, there were significant changes in the expression of two intracellular proteins that are enriched in the dendritic spine and link dopamine receptors to other effector pathways. The most dramatic change was noted for the transcript that encodes calcyon. Calcyon is a recently identified protein found to interact with the D
1 receptor, which may facilitate D
1 receptor-mediated increases in intracellular calcium. Yeast two-hybrid and coimmunoprecipitation studies have shown that calcyon binds intracellular C-terminal residues of the D
1 receptor, and immunocytochemical studies showed that this protein is widely expressed throughout the brain
(9). By binding a C-terminal region of the D
1 receptor, calcyon appears to mediate calcium mobilization by permitting “crosstalk” between D
1-coupled G
s proteins and G
q/11 protein-coupled receptors, including metabotropic glutamate and muscarinic cholinergic receptors
(9). D
1 receptor stimulation alone does not affect calcium levels, but application of a D
1 agonist after stimulating P2Y purinergic or M
1 muscarinic receptors triggered a large increase of intracellular calcium
(9). Calcyon has also been shown to regulate the affinity state of the D
1 receptor
(63). Therefore, calcyon may serve several functions in the neuron, including regulating D
1 receptor affinity for agonists as well as the integration of D
1-mediated signaling with G
q11 protein- coupled receptors. A recent study found that levels of expression of calcyon protein (expressed both per total protein and per neuronal DNA) were significantly increased in the dorsolateral prefrontal cortex of patients with schizophrenia
(13). These results in the prefrontal cortex are consistent with our present data of a significant elevation of calcyon mRNA in the thalamus in schizophrenia.
Spinophilin was also significantly increased in the thalamus in schizophrenia. Yeast two-hybrid has been used to demonstrate an interaction between the third intracytoplasmic loop of the D
2 receptor and spinophilin, a protein enriched in dendritic spines and known to bind PP-1 and F-actin
(64,
65). Spinophilin is also widely expressed in the brain but is particularly enriched in the hippocampus, striatum, and thalamus
(64). Spinophilin may function, in part, as a protein phosphatase-1-targeting protein to concentrate protein phosphatase-1 in dendritic spines, where it would be in close proximity to postsynaptic targets such as
N-methyl-
d-aspartic acid (NMDA) and AMPA glutamate receptors and calcium/calmodulin-dependent kinase II (CamKII)
(12,
64). Spinophilin may also impact synaptic transmission by regulating dendritic spine number and density
(64,
66) by targeting protein phosphatase-1 to the spine microfilament network, which is dynamically modified by protein phosphatase-1 activity
(67). Data from a spinophilin knockout mouse support both of these possibilities, as these animals show both changes in glutamatergic transmission and spine density
(66). Although spinophilin has been found to interact with the D
2 receptor in vitro, this interaction has not yet been established in vivo
(11). Since spinophilin has several binding sites, including a site to bind F-actin, protein phosphatase-1, and D
2, as well as a PDZ domain, it may serve as a scaffold-adapter protein for G-protein coupled receptors, which in turn could link receptors to the cytoskeleton and coordinate their associated signaling pathways. A recent study that examined levels of expression of spinophilin in the dorsolateral prefrontal cortex in schizophrenia did not reveal any significant differences
(13). Our present data indicating increased spinophilin mRNA in the thalamus in schizophrenia suggest that brain region-specific alterations in levels of expression of spinophilin may occur in schizophrenia.
DARPP-32 levels were unchanged in schizophrenia. D
1-type dopamine receptors primarily couple to G
s–G proteins to stimulate cAMP formation, which activates cAMP-dependent protein kinase A. Protein kinase can phosphorylate numerous intracellular proteins, including the phosphoprotein DARPP-32, which plays a central role in neuronal function, particularly in brain regions that receive rich dopaminergic input
(8). Many neurotransmitters, including dopamine, modulate the phosphorylation and/or dephosphorylation state of DARPP-32
(68). The phosphorylated form of DARPP-32 is a potent inhibitor of protein phosphatase-1, the predominant phosphatase used in neurons, which is particularly enriched in the postsynaptic density
(69). The DARPP-32/protein phosphatase-1 cascade controls the phosphorylation states and activity of myriad downstream effectors and therefore exerts a powerful effect on neuronal function
(8).
A previous study revealed that DARPP-32 expression was significantly reduced in the dorsolateral prefrontal cortex in schizophrenia
(14). The same study also revealed that DARPP-32 was not reduced in patients with Alzheimer’s disease who had been treated with haloperidol, suggesting that the decrease in DARPP-32 observed in schizophrenia is unlikely to be the result of antipsychotic treatment alone. Our finding of no change of DARPP-32 mRNA in schizophrenia is perhaps not unexpected. DARPP-32 is likely not transcriptually regulated in schizophrenia. Instead, changes in this molecule are likely translational or particularly at the level of phosphorylation state.
Our study has several limitations. These data are from elderly patients with schizophrenia, so generalization of our findings to younger patients should be made with caution. Another potential confounding variable that must be considered in this study, as in any study of postmortem brain in schizophrenia, is the possibility that any findings are due to past antipsychotic exposure. It is unlikely that our significant results are due to drug effects. Several studies in the macaque have examined the effects of haloperidol on expression of both calcyon and spinophilin, although only in the cortex. Chronic antipsychotic treatment did not alter expression of calcyon in the prefrontal cortex
(13). On the other hand, spinophilin levels are decreased in the cortex following haloperidol treatment
(13,
70), opposite to our findings in the thalamus in schizophrenia. In addition, we did not detect any positive correlations between expression of these transcripts and antipsychotic exposure. While we cannot completely rule out the possibility that our findings are associated with antipsychotic treatment, these data strongly suggest otherwise.
Many studies of neurotransmitter-mediated abnormalities in schizophrenia have focused on cell membrane expressed molecules, particularly neurotransmitter receptors. Our results suggest that there are dopaminergic abnormalities in the thalamus in schizophrenia but that they occur at the level of intracellular proteins that modulate dopamine receptor-related intracellular signaling events. These proteins, calcyon and spinophilin, link dopamine receptor-signaling pathways to other neurotransmitter receptors
(11,
12,
71,
72), so our findings suggest that in schizophrenia, the intracellular integration of dopamine signaling with other neurotransmitter systems in the dendritic spine is abnormal in the thalamus. Specifically, while calcyon and spinophilin appear to mediate intracellular interactions between dopamine and other neurotransmitters, both have been shown to promote dopamine-glutamate synergies
(12,
71,
72). Our current data, along with our previously published observations of altered expression of NMDA receptors
(29) and NMDA receptor-interacting intracellular molecules like PSD95
(33) in these same subjects, suggest that the neurochemical disturbances in thalamic circuits in schizophrenia may involve abnormalities of the molecules that integrate glutamatergic and dopaminergic signaling pathways in thalamic neurons.