Bipolar disorder is a serious and chronic psychiatric illness whose neuropathology is still largely unknown. The involvement of prefrontal brain regions in bipolar disorder has been replicated by various research groups and is supported by several lines of evidence. In vivo structural neuroimaging studies have reported decreased prefrontal cortical volumes among bipolar patients
(1–
3). Postmortem analysis of this region has revealed specific abnormalities such as decreased neuronal and glial density
(4). Investigations employing functional magnetic resonance imaging, positron emission tomography, or single photon emission computed tomography all have consistently reported decreased frontal glucose metabolism
(5,
6) and lower frontal blood flow in bipolar patients, mostly during depressive episodes
(3,
7).
Discrete prefrontal cortical regions have been investigated in bipolar disorder patients, with interesting although sometimes conflicting results. Drevets et al.
(8) reported decreased volume and functioning of the subgenual prefrontal cortex among bipolar patients, familial subtype. A postmortem study
(9) found subgenual abnormalities, mainly glial density reduction, in this same population. Our group, however, has failed to identify volumetric abnormalities in this region among mood disorder patients
(10). Hypoactivation of orbital and rostral prefrontal cortices has been reported in manic patients
(11), although other investigators have not found any rCBF abnormalities in bipolar disorder
(12).
The dorsolateral region of the prefrontal cortex, encompassing Brodmann’s areas 9 and 46
(13), is believed to play a major role in decision making
(14) and working memory
(15,
16), acting as an interface between cognition and emotion
(17–
19). Utilizing proton magnetic resonance spectroscopy (
1H-MRS), a methodology that provides information on the brain content of several metabolites in vivo, Winsberg et al.
(20) observed significantly decreased
N-acetylaspartate (NAA) in the dorsolateral prefrontal cortex of unmedicated adult bipolar patients. NAA is found primarily in neurons
(21) and is a nonspecific marker of neuronal integrity
(22). On the other hand, Moore et al.
(23) reported a significant increase in NAA levels after 4 weeks of lithium treatment in bipolar patients, possibly as a consequence of lithium’s neurotrophic actions
(24). However, most neurobiological studies of affective disorder were conducted with adult patient groups. For instance, the average age of subjects in the Winsberg et al.
(20) and Moore et al.
(23) studies was 37.9 and 36.3 years, respectively. Therefore, it is not clear if the reported abnormalities were present since the early stages of the illness or whether they might be related to illness progression or medication use.
The purpose of the present study was to investigate metabolite abnormalities in the dorsolateral prefrontal cortex of bipolar disorder youth. A younger patient population was chosen in order to minimize the effects of confounding variables such as long-term medication usage. We hypothesized that lower NAA levels would be found among patients with bipolar disorder, representing abnormal prefrontal processes of possible neurodevelopmental origin that are already present at the early stages of the disease. Moreover, levels of choline-containing compounds in this same region would allow us to indirectly evaluate membrane phospholipid metabolism and signaling of the phosphatidylcholine system, providing information on neuronal membrane functioning.
Method
Subjects
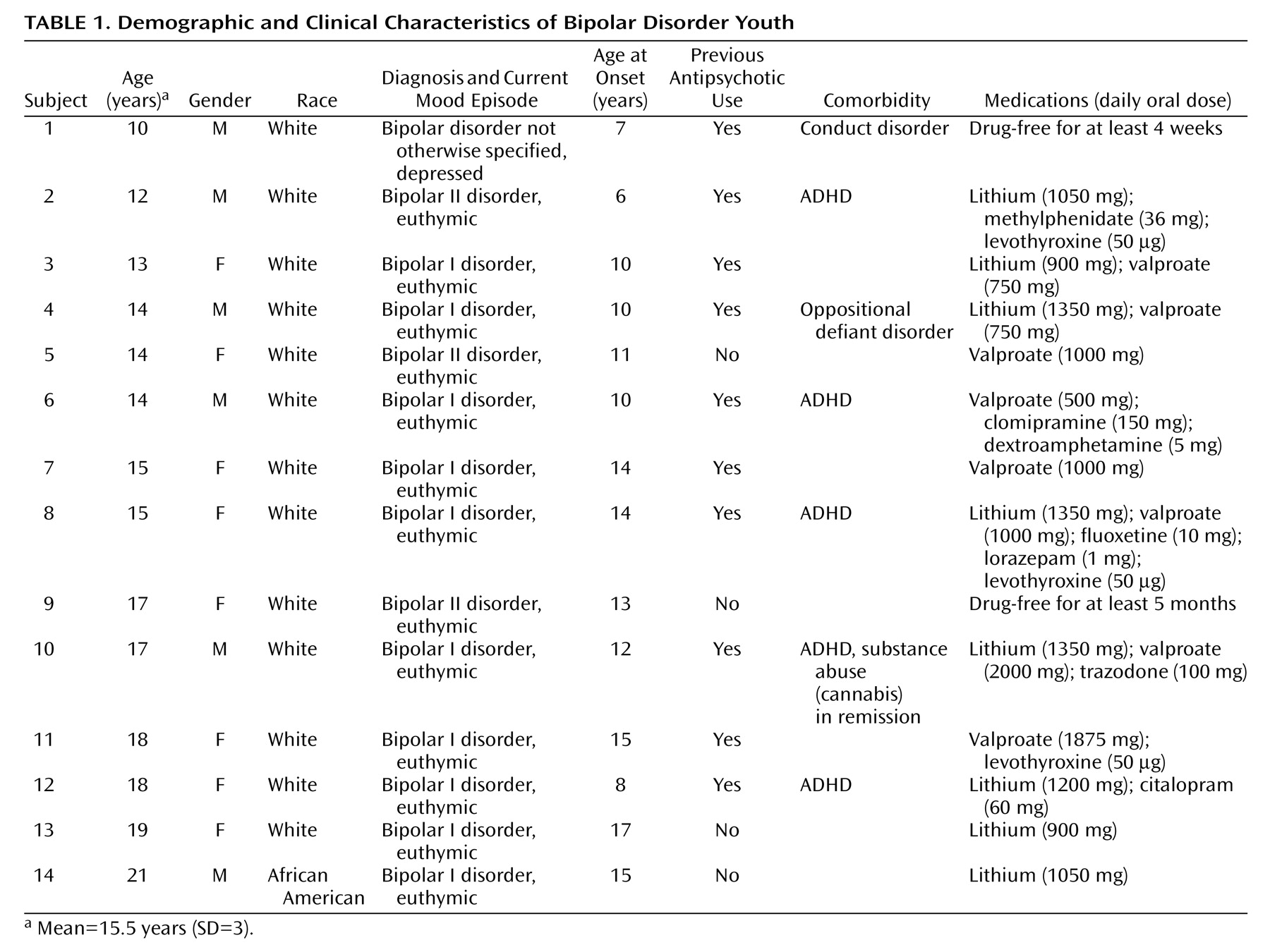
Thirty-two subjects were studied, of whom 14 were DSM-IV bipolar disorder patients (
Table 1) and 18 were healthy comparison subjects (mean age=17.3 years [SD=3.7, median=18, range=11–21]; female: N=7, male: N=11; African American: N=3, Caucasian: N=15). After having understood all issues involved in participation in the study protocol, all subjects and their parents or legal representatives provided signed informed study consent. This research study was approved by the University of Pittsburgh Biomedical Institutional Review Board. The patients were recruited at the outpatient facilities of the University of Pittsburgh Medical Center or through advertisements in the local media. The inclusion criteria were age between 10 and 21 years and a diagnosis of bipolar disorder, any subtype, in any mood state. All patients met DSM-IV criteria for bipolar disorder. For patients 10–17 years old, diagnosis was determined with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL)
(25). Patients 18–21 years old were assessed with the Structured Clinical Interview for DSM-IV (SCID), patient edition
(26). Information about family history of psychiatric disorders, age at onset of illness, length of illness, number of previous DSM-IV affective episodes, number of weeks receiving lithium treatment, current lithium dose, and medication history was retrieved from psychiatric interviews with the patients and medical charts.
All subjects had normal physical examination results and no history of neurological problems. The patients did not have any comorbid psychiatric diagnoses with the exception of ADHD (five of 14), conduct disorder (one of 14) and oppositional defiant disorder (one of 14). At the time of the study, one patient was in a depressive episode and 13 were euthymic. Only two patients were drug-free, and 12 patients were receiving medication treatment at study entry. Ten patients had a history of previous antipsychotic usage, whereas four patients had no such history. Only one patient did not have a positive family history of mood disorders in a first-degree relative. First-degree relatives were considered positive for mood disorders if they ever received a diagnosis of unipolar or bipolar disorder by a psychiatrist, as ascertained by patient and relative reports or available medical records. Furthermore, the patients did not have any current medical problems or alcohol/substance abuse in the 6 months preceding the study. One patient had a previous history of substance abuse (cannabis) that had been in remission for more than 6 months before the study, and no patient had a lifetime history of substance dependence (
Table 1).
We could not ascertain the number of previous affective episodes for one patient. Our patient group had the following clinical characteristics: length of illness: mean=3.79 years (SD=2.39, median=3, range=1–10); age at first affective episode: mean=11.71 years (SD=3.24, median=11.5, range=6–17); number of previous affective episodes: mean=4.85 (SD=2.34, median=3, range=2–9).
Healthy subjects had no DSM-IV axis I disorders, as determined either with the SCID or K-SADS-PL depending on subject age. Comparison subjects also had no current medical problems, no lifetime history of substance dependence or substance abuse in the 6 months preceding the study, and no history of psychiatric disorders among first-degree relatives.
MRS Method
In vivo 1H MRS was conducted on a GE Signa Imaging System (General Electric Medical Systems, Milwaukee), at field strength of 1.5 T. A three-dimensional spoiled gradient-recall acquisition was performed in the coronal plane (TR=25 msec, TE=5 msec, flip angle=40°, field of view=24 cm, slice thickness=1.5 mm, number of excitations=1, matrix size=256×192) to obtain 124 images covering the entire brain. A double spin-echo sequence was also used to obtain T2 and proton density images in the axial plane to screen for neuroradiological abnormalities.
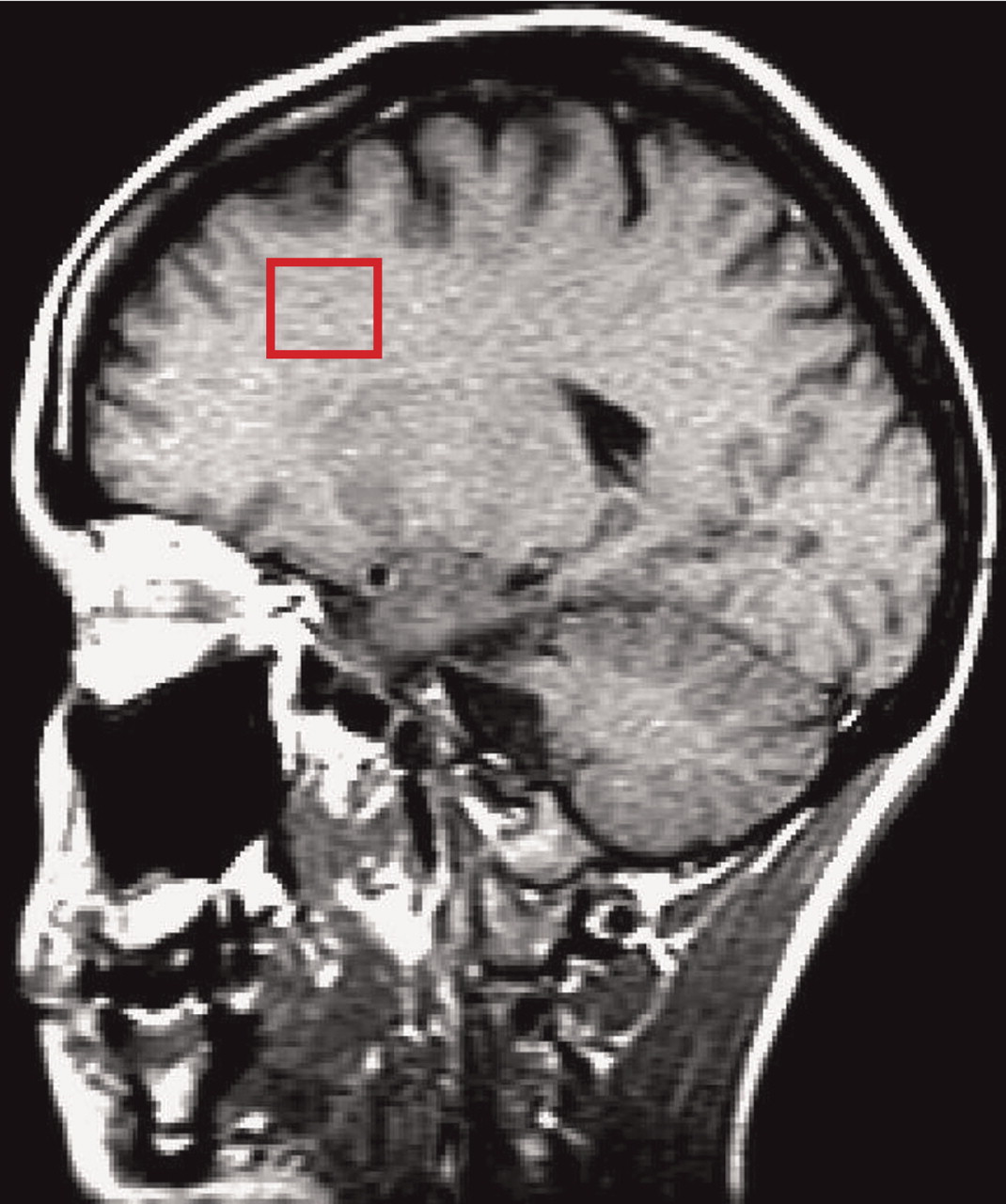
The single-voxel short TE MRS data were collected with a STEAM sequence (TE=20 msec, TM=13.6 msec, TR=1.5 seconds, bandwidth=2 kHz, 2,048 complex data points, 300 acquisitions, voxel dimension=2.0×2.0×2.0 cm
3). This 8-cm
3 voxel was placed in the left dorsolateral prefrontal cortex (
Figure 1), which was identified on the set of sagittal and coronal MR images using standard anatomical atlases
(28,
29). A second STEAM spectrum was collected without water suppression (16 acquisitions). On the basis of a semiautomated histogram method
(30,
31), the percent volume of gray matter, white matter, and CSF within the MRS voxels was estimated from the three-dimensional spoiled gradient-recall acquisition data by using the NIH Image software package, version 1.62 (National Institutes of Health, Bethesda, Md.). The intraclass correlation coefficients for the histogram measurements obtained by two independent raters (P.B., M.A.N.) in a group of 10 subjects were 0.94 for gray matter, 0.94 for white matter, and 0.91 for CSF.
The MRS postprocessing and quantification steps were 100% automated. The unsuppressed water spectrum was used to correct for any eddy current effects. No apodization was applied, and any residual water signal was removed by using the operator-independent, singular-value-decomposition-based method
(32). We investigated three major metabolites: NAA, creatine plus phosphocreatine, and choline-containing molecules (mostly glycerophosphocholine plus phosphocholine
[33]). Five Gaussian damped sinusoids (NAA at 2.01 ppm, creatine plus phosphocreatine at 3.02 and 3.93 ppm, glycerophosphocholine plus phosphocholine at 3.21 ppm, and
myo-inositol at 3.54 ppm) were used to model the in vivo data in the time domain using the Marquardt algorithm. To ensure that the signals of overlapping and of lesser amplitudes (i.e., metabolites with multiplet structures and macromolecules) had negligible influence on the fitting of the singlets, the first 37 msec of the free-induction decay signal were omitted in the fitting, which has been shown to reliably and accurately quantify NAA, creatine plus phosphocreatine, and glycerophosphocholine plus phosphocholine
(34). The unsuppressed water signal along with the appropriate correction factors was applied to obtain absolute quantification values with units of mmol/kg wet weight.
Statistical Analyses
All analyses were conducted by using the SPSS for Windows software, version 10.0.5 (SPSS Inc., Chicago), and two-tailed statistical significance level was set at p<0.05. Analysis of covariance (ANCOVA) with age and gender as covariates was performed to compare metabolite concentrations between groups. Pearson’s correlation coefficients were used to examine the effects of age and specific clinical variables on the metabolite concentrations. ANCOVAs with Scheffe post hoc tests were performed to evaluate the effects of gender on metabolite measures. The nonparametric Mann-Whitney U test was used to perform comparisons between patient subgroups, since the group sizes involved were relatively small.
Results
Bipolar disorder patients and healthy comparison subjects did not differ with regard to age (F=2.09, df=1, 30, p=0.14), gender (χ2=1.05, df=1, p=0.30), race (χ2=0.65, df=1, p=0.42), or handedness (χ2=1.81, df=1, p=0.18).
Within the 8-cm3 voxel, there were no significant differences between bipolar patients and healthy subjects in volumes of gray matter (mean=3.68 cm3 [SD=0.51] and 3.66 cm3 [SD=0.73], respectively; F=1.28, df=1, 29, p=0.91), white matter (mean=3.87 cm3 [SD=0.55] and 3.93 cm3 [SD=0.82]; F=1.56, df=1, 29, p=0.84), or CSF (mean=0.08 cm3 [SD=0.12] and 0.07 cm3 [SD=0.04]; F=1.05, df=29, p=0.73).
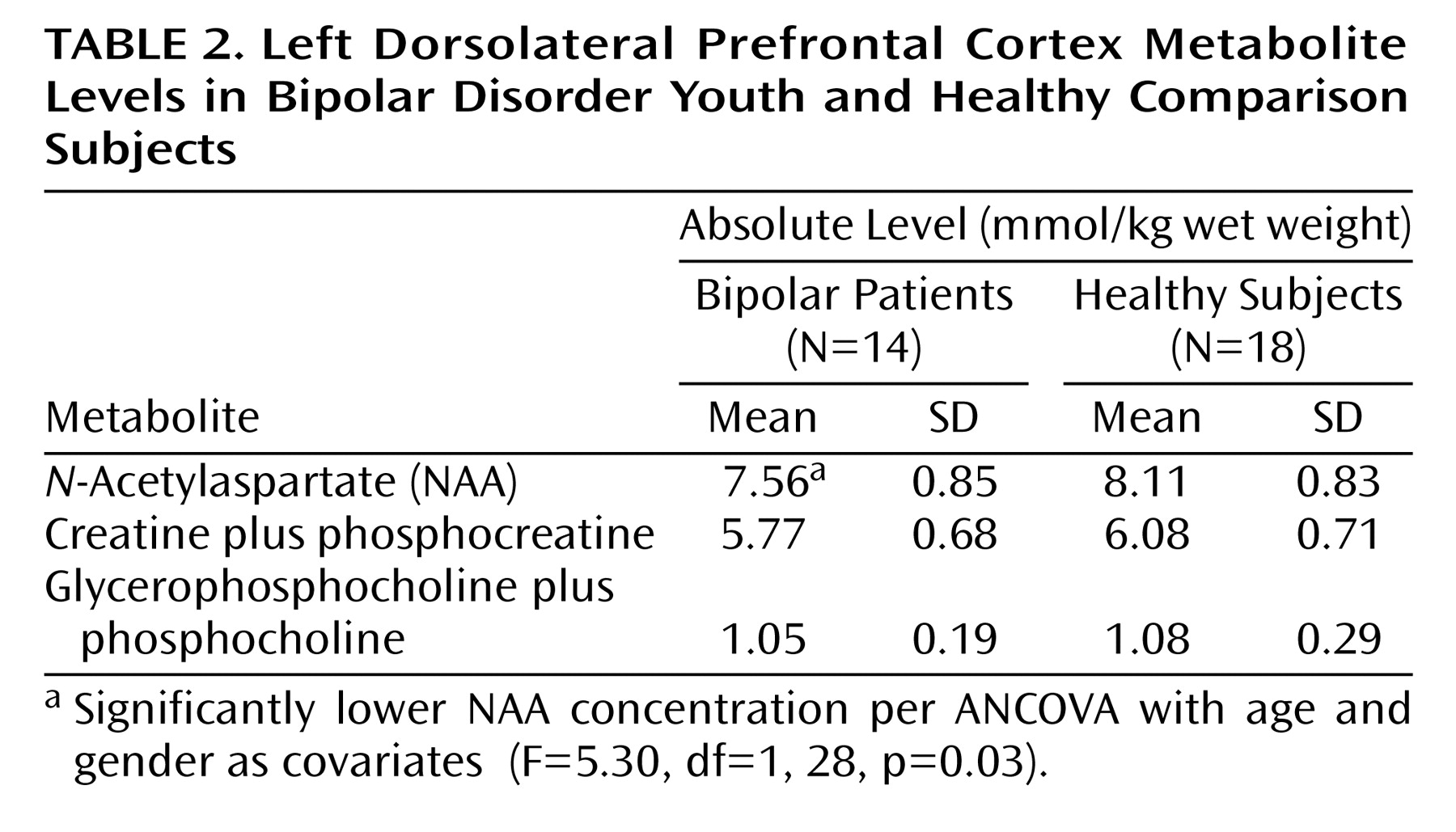
The
1H metabolite levels examined are presented in
Table 2. When comparing the two groups, we found that bipolar patients had significantly decreased NAA levels relative to healthy subjects. Bipolar patients also tended to have decreased creatine plus phosphocreatine levels relative to healthy subjects (ANCOVA F=3.22, df=1, 28, p=0.08). In contrast, no between-group differences were observed for glycerophosphocholine plus phosphocholine levels (ANCOVA F=0.002, df=1, 28, p=0.96).
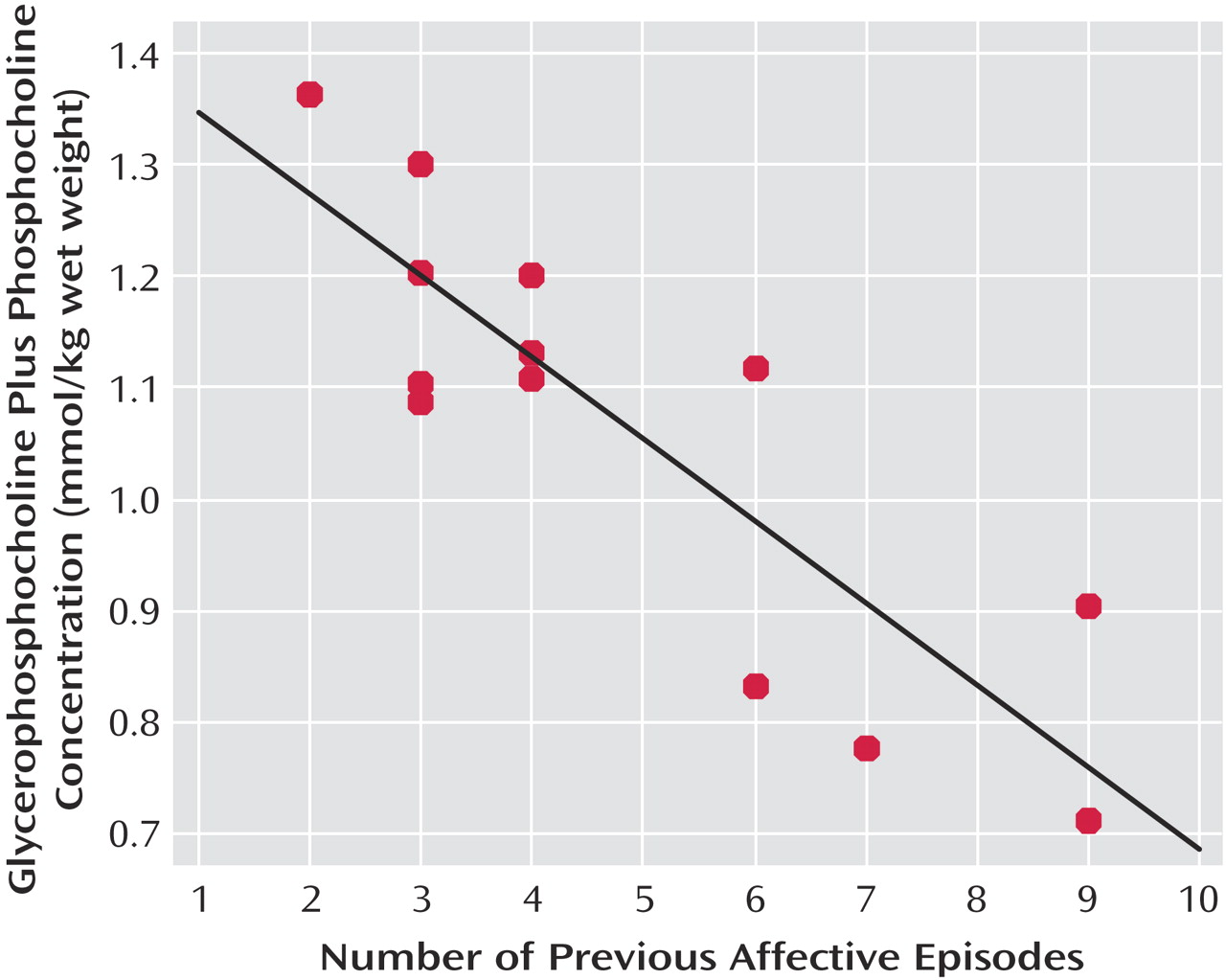
No significant correlation was observed between age and any metabolite concentration in bipolar patients. Moreover, no significant correlation was found between any metabolite concentration and age at first affective episode, length of illness, lithium dose, or valproate dose. On the other hand, we observed a significant inverse correlation between number of previous affective episodes and glycerophosphocholine plus phosphocholine concentration (
Figure 2).
Discussion
We report here having found significantly lower NAA levels in the left dorsolateral prefrontal cortex of bipolar disorder youth relative to healthy comparison subjects. Our results provide indirect support for the hypothesis that neuronal dysfunction, or decreased neuronal density, is present in the prefrontal cortex of bipolar patients already at early stages of the illness. On the basis of only cross-sectional MRS observations it is not possible to determine whether decreased NAA in the dorsolateral prefrontal cortex of young patients represents a general underdevelopment of dendritic arborizations and synaptic connections or degenerative/atrophic processes that may progress during the course of illness.
The results of this study are consistent with reports of decreased NAA levels in the dorsolateral prefrontal cortex of adult bipolar patients
(20,
35) as well as postmortem data demonstrating decreased neuronal density in that region in adult mood disorder patients
(4,
36). Furthermore, another MRS study of pediatric bipolar patients who were children of a bipolar parent, reported by Chang et al.
(37), also showed similar findings, although only for the right dorsolateral prefrontal cortex. Some important differences between our study and the one by Chang et al.
(37) must be pinpointed. First, our patients were older (mean age=15.5 [SD=3] versus 12.6 years [SD=2.9]), more evenly distributed regarding gender (percentage of girls was 57% versus 13%), and presented less comorbidity (36% versus 87% for comorbid ADHD) than their bipolar disorder patients. On the other hand, Chang et al.
(37) included only patients with bipolar I disorder and a history of bipolar disorder in at least one first-degree relative, whereas our inclusion criteria was less stringent. Our study group included patients with bipolar disorder type II or not otherwise specified, and patients who had either unipolar or bipolar disorder diagnosed in a first-degree relative were considered to have a positive family history. There were also considerable technical differences between our protocols. First, we measured absolute metabolite levels instead of Cr ratios, which might have helped us to minimize potential confounders. Second, we utilized STEAM instead of PRESS to acquire the MRS data and ended up with reliable spectra for only three peaks (NAA, creatine plus phosphocreatine, glycerophosphocholine plus phosphocholine), whereas Chang et al.
(37) also measured
myo-inositol levels in addition to those three metabolites. Last, and most important, we did not measure the level of any metabolites in the right dorsolateral prefrontal cortex, whereas Chang et al.
(37) examined both right and left dorsolateral prefrontal cortex. Thus, we were not able to report in our study whether decreased NAA in the dorsolateral prefrontal cortex was a bilateral phenomenon or restricted to the left side only. It is not clear if any of the differences in patient selection or data acquisition might account for the differences in the final results between our study and the one by Chang et al.
(37). In any case, the lack of a bilateral dorsolateral prefrontal cortex measurement is one of the main limitations of our study.
Some other limitations should also be considered. It is difficult to examine the influence of clinical variables on prefrontal NAA levels, mostly due to our reduced study group size. For instance, our bipolar group had only three bipolar II patients, which limited our ability to compare whether the changes in NAA concentration were related to any specific bipolar subtype. Furthermore, we did not identify in the bipolar subjects any correlation between NAA levels and age, length of illness, or number of previous affective episodes. Along the same lines, other phenomenological characteristics that might have influenced our final results (e.g., history of psychotic symptoms or suicide attempts) were not addressed given our small patient group size. Moreover, the large majority of our patients were taking mood stabilizers and other medications such as antidepressants and stimulants, restricting our capacity to rule out possible pharmacological effects. Studies with a larger number of subjects, a prospective design, and larger numbers of untreated subjects will be needed to address the influence of medications on NAA levels in bipolar patients.
Another limitation of our study concerns the lack of other comparable brain structures. It is believed that mood regulation involves several cortical and subcortical regions and that dysfunction in one area might lead to abnormalities in other connected structures. Since we examined only a single region, we were not able to verify if decreased NAA is also found in other brain structures of interest in child and adolescent bipolar patients.
Our method did not allow a reliable quantification of other metabolites reported in previous proton MRS studies, such as
myo-inositol, glutamate, and glutamine. On the other hand, we were able to obtain absolute concentration of NAA, creatine plus phosphocreatine, and choline-containing compounds (glycerophosphocholine plus phosphocholine), which represents an advantage over ratios in terms of sensitivity and eliminates an important confounding factor.
1H MRS, in contrast with
31P-MRS, is limited when discriminating the specific components that contribute to the choline peak. Nonetheless, it is believed that glycerophosphocholine plus phosphocholine reflects either membrane phospholipid metabolism or the rate of signaling of the phosphatidylcholine system
(38). There are reports of increased choline in the anterior cingulate
(39) and basal ganglia
(40–
42) among adult bipolar patients and also increased choline in the left dorsolateral prefrontal cortex
(43) and orbitofrontal cortex
(44) in depressed adolescent patients. On the other hand, Castillo et al.
(45) have not found any temporal or frontal changes in choline-containing molecules or NAA in children with bipolar disorder, and Cecil et al.
(35) reported decreased choline concentration in the frontal lobe of adult bipolar patients.
We did not find any significant difference in glycerophosphocholine plus phosphocholine concentration between bipolar patients and healthy subjects, but we observed a significant negative correlation between choline-containing molecules and the number of previous affective episodes. There is a possible bias that may explain these findings. Patients with several previous affective episodes were potentially exposed to different classes of medication and probably were treated for longer periods. Thus, patients with multiple affective episodes have a pharmacological history that is substantially distinct from patients with only a few illness episodes. However, there is evidence that lithium does not alter the brain concentration of choline-containing molecules
(41,
46,
47), although response to antidepressants seems to be related to changes in the brain levels of this metabolite
(48). Moreover, it is not clear if other medications such as antipsychotics, stimulants, and anticonvulsants exert any influence on the levels of choline-containing molecules. Thus, we are not able to rule out medication effects as an alternative explanation for such findings. Nonetheless, an inverse correlation of choline-containing molecules and number of affective episodes may represent neurodegenerative processes occurring in the prefrontal cortex. To the best of our knowledge, this is the first time such a relationship has been reported. Replication in larger samples with stricter medication profiles is needed to assess whether these findings have relevance for illness progression.
Children and adolescent patients have been largely overlooked in MRS investigations. Fortunately, this trend has started to change in recent years, since the examination of younger patients allows us to minimize potentially confounding factors commonly found in adult patients, such as long-term medication effects. Nonetheless, we wish to underscore the preliminary nature of the present study. A replication in a larger study group with medication-naive or first-break bipolar patients is warranted. Nevertheless, in the context of most other MRS studies that investigated only older subjects, our report suggests that youth with bipolar disorder exhibit abnormalities in the dorsolateral prefrontal cortex that are similar to those found in adult patients.