Neurological soft signs refer to subtle neurological abnormalities comprising deficits in sensory integration, motor coordination, and sequencing of complex motor acts
(1). A considerable body of research has established that neurological soft signs are more prevalent in schizophrenia patients, including first-episode cases, than in healthy subjects
(2). Studies with neuroleptic-naive first-episode patients have demonstrated that neurological soft signs are present before medication exposure, thus they are thought to be an intrinsic feature of schizophrenia
(3,
4). This notion is supported by findings of neurological soft signs in high-risk subjects (i.e., relatives of schizophrenia patients and unaffected co-twins of monozygotic twin pairs discordant for schizophrenia [
5–
7]). These studies found that relatives take an intermediate position between healthy and schizophrenia subjects.
Given these results, neurological soft signs can be interpreted as an expression of genetic liability toward the disease as have been presented in Meehl’s concept of “schizotaxia”
(16) in which dysdiadochokinesia—among others—constitutes a trait-like marker of a baseline defect (“hypokrisia”). To further understand fluctuations in the level of neurological soft signs and in particular their increase with acute symptoms of schizophrenia, Huber’s hypothesis of “process activity”
(17) may serve as a complementary concept. Huber differentiates reversible and irreversible symptoms, the former representing functional states of the active disease process that may remit with clinical stabilization. From his pneumencephalographic study
(18) he concluded that once the active process persists, structural and psychopathological deteriorations run a parallel course toward an irreversible deficit. Hence, process activity does not refer to a static concept but addresses the variability of the clinical course.
Drawing on the outlined concepts, we hypothesized that 1) neurological soft sign scores would increase during acute phases of the illness and return to baseline values with stabilization through treatment (possibly reflecting the activity of the disease process); 2) a decrease in neurological soft sign levels would be a favorable prognostic criterion; and 3) even in patients with a remitting course and favorable outcome, neurological soft signs would remain increased relative to healthy comparison subjects as an expression of genetic liability or schizotaxia.
Results
Demographic and Clinical Data
Patients and comparison subjects were comparable with respect to age, gender, and education. Although eight patients and only one healthy subject had a family history of psychiatric disease, this difference did not reach significance level.
Patients’ initial assessment revealed the following diagnoses: schizophrenia (N=20), schizoaffective disorder (N=2), schizophreniform disorder (N=16), and psychosis not otherwise specified (N=1). At the follow-up evaluation, a diagnostic shift was seen in 16 cases: from schizophreniform disorder to schizophrenia (N=14), from psychosis not otherwise specified to schizophrenia (N=1), and from schizophrenia to schizoaffective disorder (N=1). During the follow-up period, 33 individuals adhered to psychiatric treatment regularly. Thirty-one patients received continuous treatment with atypical antipsychotics (mean dose=297.7 mg/day in chlorpromazine equivalents [SD=35.5]); four of these patients were prescribed a mood stabilizer or an antidepressant in addition. Side effects of medication were low and unchanged at the follow-up evaluation relative to remission according to the AIMS (time 1: median=0, range=13; time 2: median=0, range=5), the Barnes Rating Scale for Drug-Induced Akathisia (time 1: median=0, range=4; time 2: median=0, range=3), and the Simpson-Angus Rating Scale (time 1: median=12, range=7; time 2: median=11, range=4).
At the follow-up evaluation, the overall Positive and Negative Syndrome Scale score (mean=52.4, SD=25.6) did not represent a significant difference from the remission score (mean=52.0, SD=12.4). The mean follow-up Strauss-Carpenter Scale score (58.4, SD=11.5) was comparable to the mean initial score (57.4, SD=11.5). During the follow-up period two patients had been continuously ill. Relapses occurred in 13 individuals, six of whom were fully recovered at the second interview. The remaining seven subjects still suffered from psychotic symptoms (N=4) or had been readmitted to the hospital (N=3). Five individuals used cannabis, four of these were also regular alcohol users. Thirty-three patients were compliant with treatment, and 31 were compliant with their medication. Ten individuals had continued their education successfully, and 20 were fully employed. Regular participation in household duties was reported by 29 subjects, regular social contacts by 24 subjects.
Neurological Soft Signs and Handedness
The patient group consisted of a similar number of right handers (N=20) and mixed handers (N=19). In the comparison group right handers (N=19) outnumbered mixed handers (N=3). While this difference between groups was statistically significant (F=8.5, df=1, 59, p<0.01), analyses did not yield a difference between the sexes within diagnostic groups.
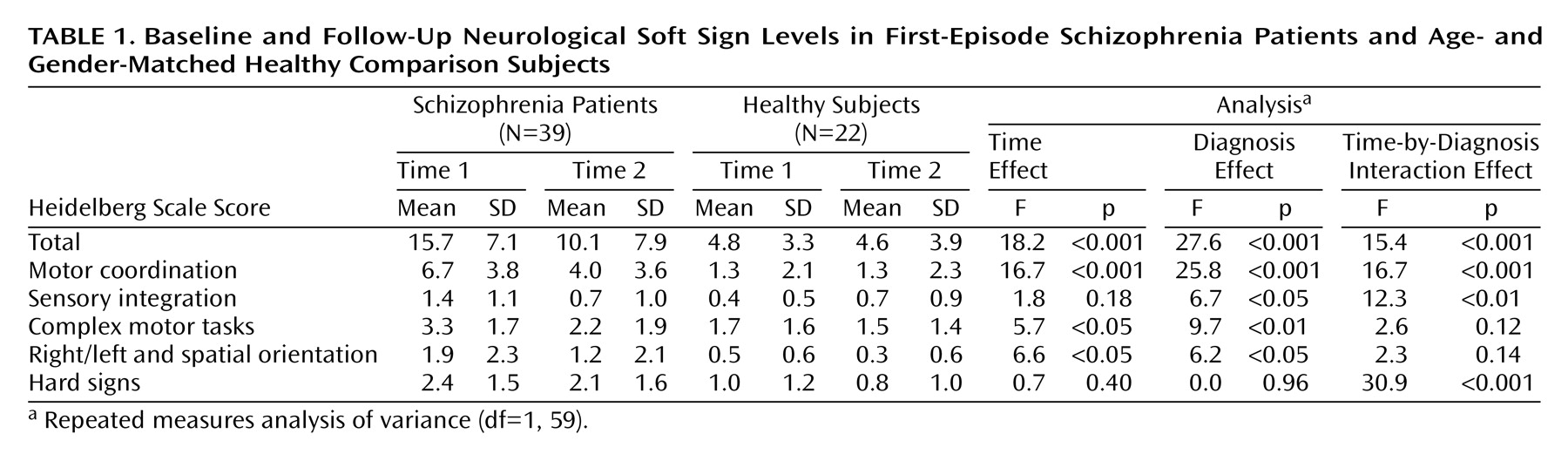
Patients’ mean initial scores on the Heidelberg Scale decreased significantly during the follow-up period (
Table 1), whereas the scores of the comparison subjects remained almost unchanged. Good test-retest reliability of the Heidelberg Scale (r=0.80, df=20, p<0.001) was seen in the healthy subjects. Neurological soft sign subscales were analyzed separately. Only for the motor coordination subscale were significant results detected for time, diagnosis, and the time-by-diagnosis interaction.
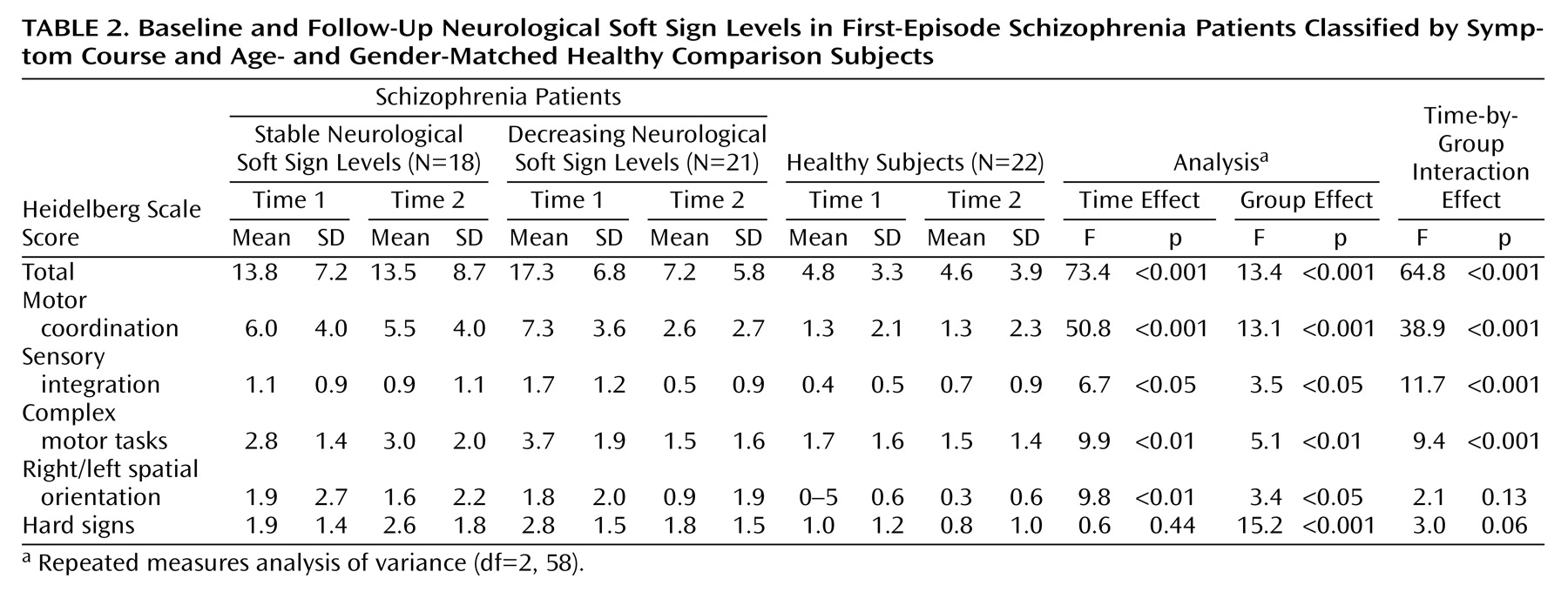
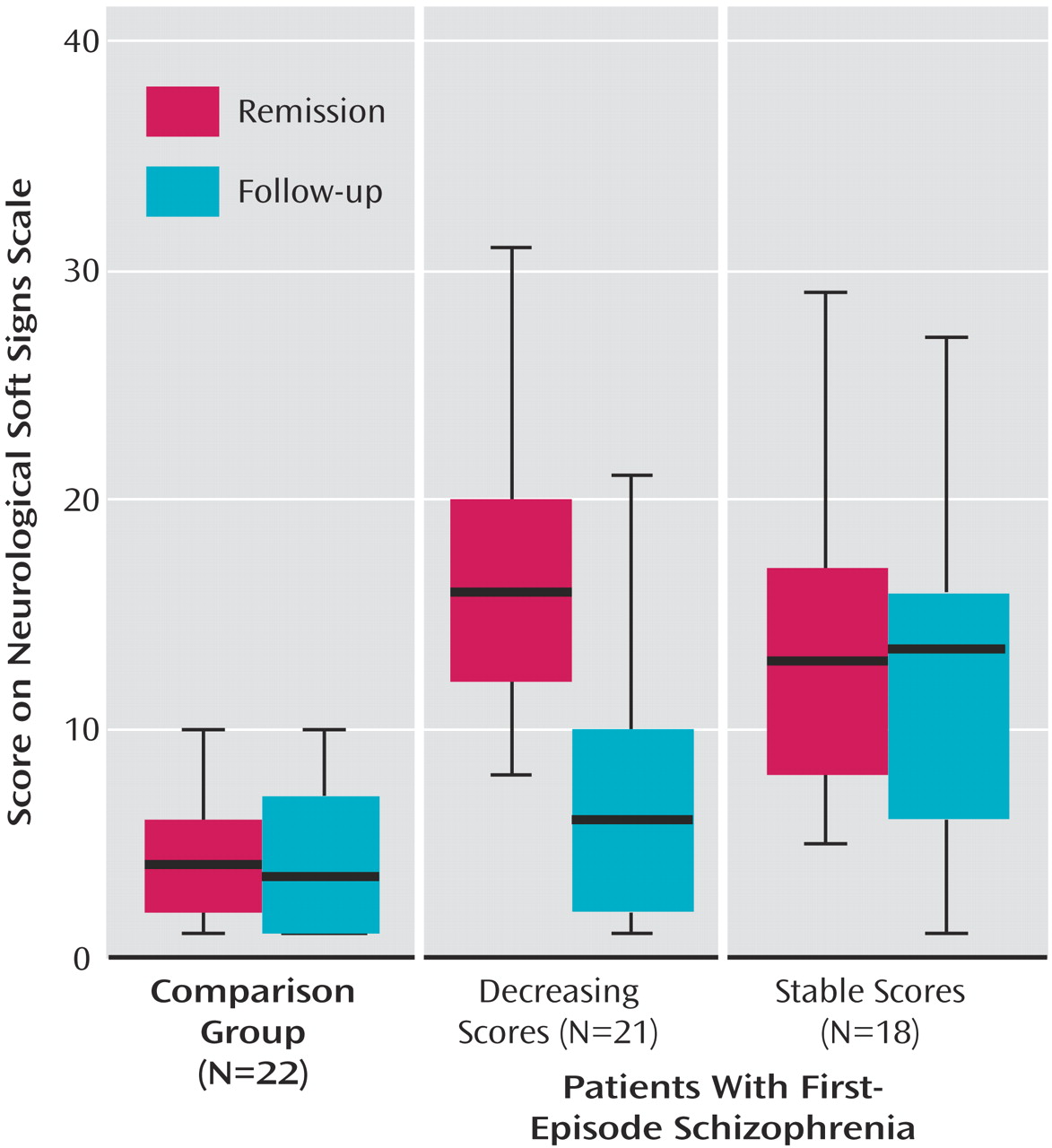
To further investigate the decrease of neurological soft signs in patients, this group was split according to the median change in neurological soft sign total scores. Subsequently, 21 patients with a pronounced neurological soft sign decrease were compared with 18 patients with stable or increasing neurological soft sign levels and to healthy subjects by means of a repeated measures ANOVA, which yielded a significant difference (
Table 2). Post hoc Duncan tests revealed that both patient groups were comparable at first assessment whereas the group experiencing a decrease in neurological soft sign levels took an intermediate position on follow-up and did not differ statistically from healthy subjects (
Figure 1).
Additional analyses of the three groups were performed for the neurological soft sign subscales (
Table 2) where significant differences emerged for motor coordination, sensory integration, and complex motor tasks.
The differences between patients and healthy subjects were not related to handedness, gender, family history of psychiatric disease, or any other sociodemographic variable.
Neurological Soft Sign Course and Clinical Measures
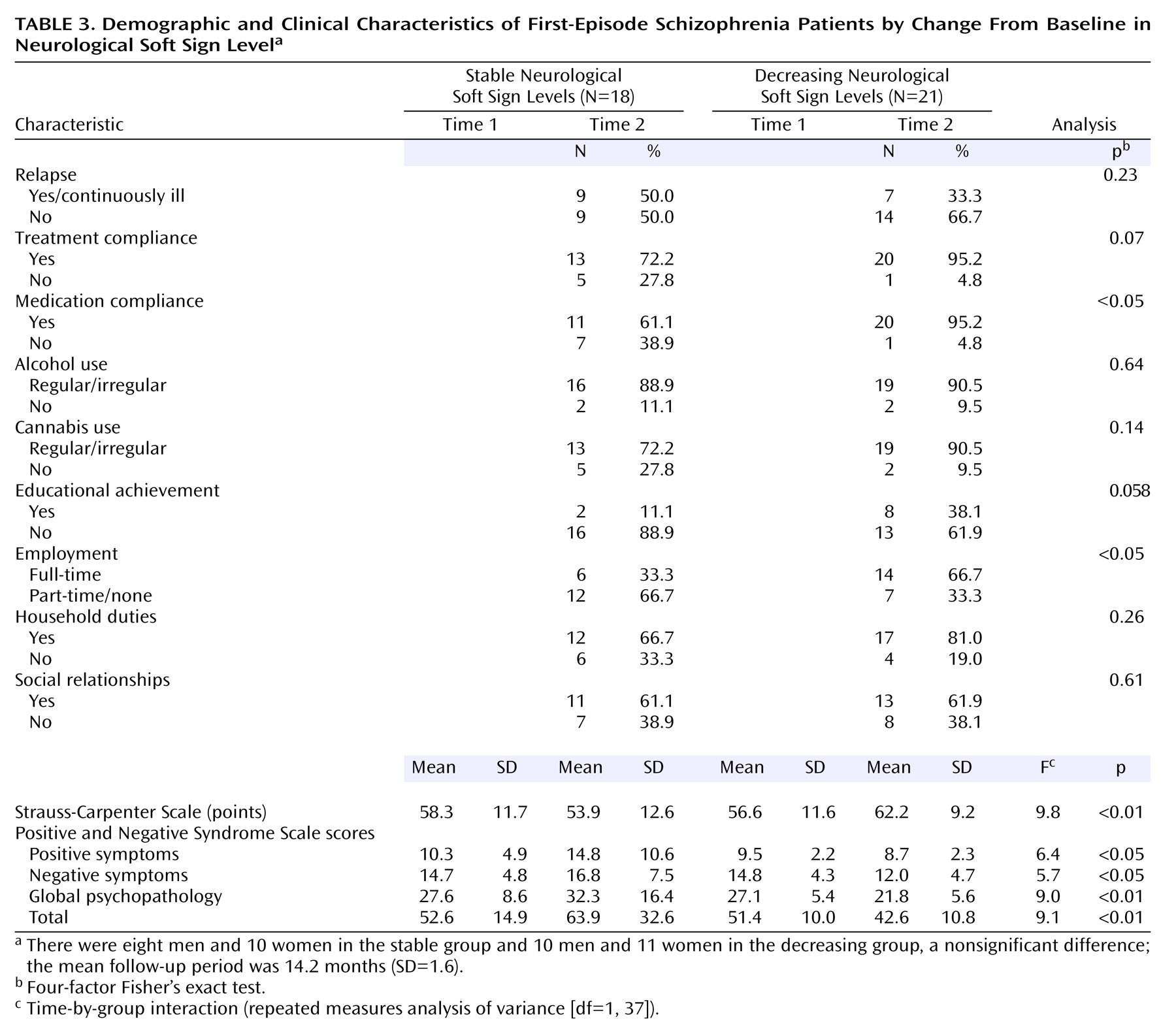
Table 3 depicts sociodemographic and clinical data for patients with decreasing and stable neurological soft sign scores. No differences between groups emerged with respect to gender, social relationships, household duties, relapse, or alcohol/cannabis use. The subgroup with decreasing neurological soft sign levels showed better compliance and educational and vocational achievement. Time-by-group interactions revealed more favorable findings for patients with decreasing neurological soft sign levels as opposed to those with stable levels in terms of scores on the Strauss-Carpenter Scale and the Positive and Negative Syndrome Scale. In addition, for the Positive and Negative Syndrome Scale there were significant main effects of group for the total score (F=5.0, df=1, 37, p<0.05), positive symptom score (F=5.0, df=1, 37, p<0.05), and global psychopathology score (F=4.3, df=1, 37, p<0.05).
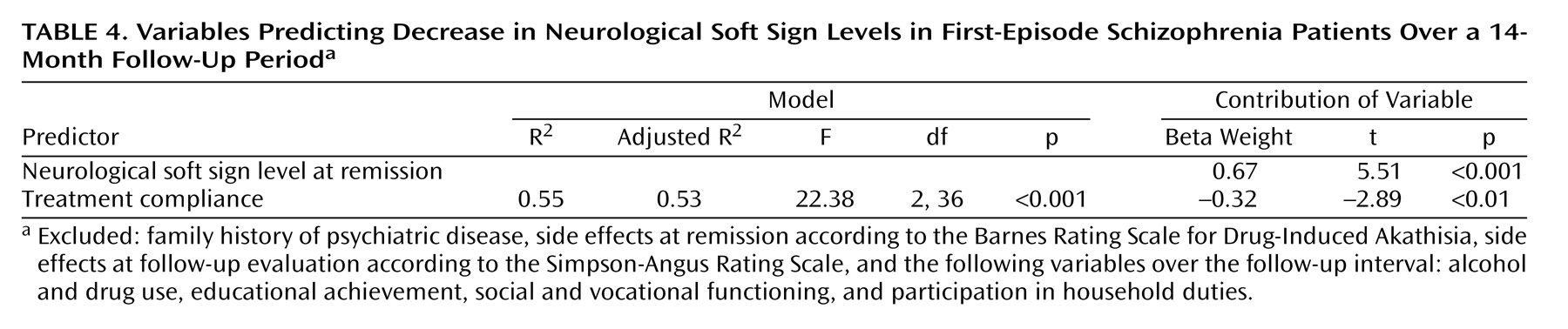
A regression analysis was calculated for neurological soft signs at the follow-up evaluation to identify predictors of neurological soft sign decrease. Variables that preceded the second assessment were entered. The analysis revealed neurological soft sign levels at remission and compliance with treatment during the follow-up period to be relevant influences (
Table 4).
Discussion
To our knowledge this is the first prospective longitudinal study to compare neurological soft signs in first-episode schizophrenia patients and healthy subjects. Whereas soft signs remained almost stable in healthy subjects, they significantly decreased in first-episode schizophrenia patients during a follow-up period of 14 months. This effect was related to better outcome. Despite the significant decrease, neurological soft signs remained elevated in patients relative to healthy subjects. Our findings support the initial hypotheses and contribute to the understanding of neurological soft signs in schizophrenia patients.
Neurological soft signs were present to a significantly greater extent in patients than in comparison subjects at both measurement points. The level of neurological soft signs at remission (time 1) is well within the range of remission scores in an earlier study by our group
(10), and follow-up neurological soft sign levels of patients with decreasing scores closely corresponded to scores of unaffected co-twins of monozygotic twins discordant for schizophrenia
(7). Our results clearly confirm the general finding of increased neurological soft sign scores in schizophrenia patients
(1,
2,
10,
26), including first-episode cases, and accord with the view that neurological soft signs range among the most consistent neurobiological characteristics of schizophrenia
(27).
During the follow-up period, neurological soft sign scores clearly decreased in patients but remained almost unchanged on a low level in healthy subjects. In particular, patients with decreasing neurological soft sign scores experienced further stabilization of symptoms and functioning, whereas clinical findings of patients with stable scores foreshadowed a chronic course. As suggested in previous studies
(10,
28), this effect arose through disturbed motor and sensory integration signs rather than orientation difficulties or hard signs. Similar findings were obtained in earlier studies by our group, namely a parallel decrease of neurological soft signs and acute symptoms in patients with remitting schizophrenia as well as in first-episode patients under initial treatment with a typical neuroleptic
(10–
12). Further support of a positive correlation between improvement in clinical status and neurological performance stems from studies describing a significant improvement of neurological soft signs during a 6-month follow-up period
(26), and more pronounced long-term deterioration in neuroleptic-free compared with medicated patients
(28). Along these lines, cross-sectional studies have consistently reported an association of neurological soft signs with increased symptom levels, poor premorbid adjustment, and unfavorable outcome
(1,
3,
10,
29) as well as with neurobiological measures such as neuropsychological deficits and structural and functional cerebral abnormalities
(10–
14). A correlation between neurological soft signs and negative symptoms was especially reported in drug-naive and medicated patients
(2). Both neurological soft signs and negative symptoms might be a consequence of dopaminergic hypoactivity. This notion accords with the difference in neurological soft signs between medication responders and nonresponders
(10,
12,
26) as well as with the finding that neurological soft signs are most prominent in chronic forms of schizophrenia
(1). The aforementioned relationships were also confirmed by our study, since neurological soft signs were significantly related to the different symptom dimensions and predictors of outcome at both measurement points. Thus, our results hint at the necessity to differentiate between patient subgroups according to their symptoms and outcome.
Since neurological soft signs are present before medication exposure, it is generally accepted that they are an intrinsic feature of schizophrenia rather than a side effect of medication
(2,
3). This view is supported by reports on spontaneous abnormal involuntary movements in schizophrenia patients, which had already been observed in the preneuroleptic era
(30). Further support for a genetic determination stems from studies on relatives of schizophrenia patients
(7,
8). Of interest in our study was that follow-up neurological soft sign scores of patients with a favorable outcome were in the range of unaffected co-twins of monozygotic twin pairs discordant for schizophrenia
(7). This is in line with evidence from studies on neuroleptic-naive patients
(3,
12,
27) and suggests that a remitting disease course leads to an increase in neurological soft sign levels during a limited period of time (i.e., an acute psychotic exacerbation) and then a return to the genetically determined baseline thereafter. These results are consistent with Meehl’s model of “schizotaxia”
(16) in which dysdiadochokinesia—i.e., a sign of dysfunctional motor coordination—is conceptualized as a marker of the baseline defect (“hypokrisia”). On the other hand, the persisting elevation of neurological soft sign scores in chronic cases may hint at a still enduring process activity reflecting premorbid changes of neurodevelopmental origin
(31) or the presence of acquired, irreversible deficits.
Thus, neurological soft signs in schizophrenia seem to adopt characteristics of both state-like and trait-like features. During the early course of acute psychosis when symptoms fluctuate, the state-like features of an active disease process
(17,
18) may be predominant. On the other hand, the trait-like features that represent the genetically determined baseline may prevail after remission of the acute illness. In conclusion, our results and the body of literature allow for the notion that neurological soft signs represent surrogate markers of the schizophrenic disease process, i.e., the process activity that is more prominent and fluctuating during the early course of the disease; in later phases of the disease the process may come to a standstill, but it may also completely resolve or deteriorate into deficit states
(17,
18).
This study may be limited by a recruitment bias. However, patients initially were included in the study consecutively as they necessitated hospital treatment. Neuroleptic medication was not standardized but restricted to atypical compounds, chosen according to the patients’ individual needs, and extrapyramidal side effects were rare and not associated with neurological soft signs. Most important, compliance with medication was a positive predictor of neurological soft sign decrease. In general, the comparability among studies is limited because of the absence of a universally accepted structured instrument. However, most instruments in use comprise a set of similar subtests, e.g., tandem gait, Romberg, diadochokinesis, finger-nose tapping, finger-thumb opposition, fist-edge palm test, Ozeretski’s test, mirror movements, graphesthesia, stereognosis, right/left orientation
(32). Therefore, overall data comparability among studies is relatively high. In spite of this methodological limitation, the evidence for a higher rate of neurological abnormalities in schizophrenia is consistent and compelling. The test-retest reliability of our Heidelberg Scale over a longer period of time was sufficient.
In summary, neurological soft signs are intrinsic to schizophrenia but their measured quantity and magnitude may serve as a surrogate marker for the activity of the disease process as well as a predictor of outcome. Overall, the assessment of neurological soft signs represents a hardly time-consuming, inexpensive, and meaningful tool in clinical psychiatry and has the potential to bridge the gulf between neurobiological research and clinical practice.