Abnormalities in brain white matter have been hypothesized to play a key role in the pathophysiology of schizophrenia
(1). Postmortem studies have identified abnormalities in the myelin sheath
(2), oligodendroglia
(3), and interstitial neurons
(4) in patients compared with healthy volunteers. If the pathophysiology of schizophrenia reflects a disturbance in the white matter, then such abnormalities might be observed by using diffusion tensor imaging. Several previous studies reported lower anisotropic diffusion
(5–
12) and a lack of normal asymmetry in this diffusion
(10) among patients with schizophrenia compared with healthy volunteers, although some studies
(13,
14) did not identify differences between subjects with and without schizophrenia in white matter integrity.
Previous diffusion tensor imaging studies may be limited by the use of patients who were chronically ill. Examination of brain white matter closer to onset of illness in schizophrenia may be important, especially given recent evidence for a progressive reduction in white matter volume over the course of the illness
(15). In this study we report a voxel-wise analysis of diffusion tensor imaging data in patients experiencing a first episode of schizophrenia or schizoaffective disorder studied early in the course of their illness. We hypothesized that these patients would demonstrate lower fractional anisotropy compared with healthy volunteers and that these effects would be greatest in the frontal and temporal lobes, consistent with hypotheses regarding frontolimbic dysfunction
(16–
18).
Method
The 10 patients included in this study (six men, four women, mean age=26.9 years, SD=4.6) were recruited from The Zucker Hillside Hospital in Glen Oaks, N.Y. Patients were experiencing a first episode of illness and had been taking antipsychotic medications for a median of 15 days. Four patients were antipsychotic-drug-naive at the time of the scan. Two patients were Caucasian, six were African American, and two were classified as “other.” Diagnoses were based on the patient edition of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)
(19) and included schizophrenia (N=5) and schizoaffective disorder (N=5). Thirteen healthy volunteers (seven men, six women, mean age=28.9, SD=6.0) also participated in this study; these subjects had no history of psychiatric illness, determined by using the nonpatient edition of the SCID
(20). Seven healthy volunteers were Caucasian, two were African American, two were Hispanic, and two were classified as “other.”
Exclusion criteria for all individuals included serious neurological or endocrine disorder, any medical condition or treatment known to affect the brain, or mental retardation defined according to DSM-IV criteria. Eight of the patients and 10 of the volunteers were dextral; two of the patients and three of the volunteers were nondextral. All procedures were approved by the local institutional review board, and written informed consent was obtained from all study participants.
Magnetic resonance imaging examinations were conducted on a 1.5-T system (GE Medical Systems, Milwaukee). Seven diffusion tensor imaging volumes were obtained for each subject, including six volumes with diffusion gradients applied along six nonparallel directions (b=800 seconds/mm2 and number of excitations=4) and one volume without diffusion weighting (b=0, number of excitations=2). Each volume consisted of 18 contiguous axial slices (slice thickness=5 mm) acquired parallel to the anterior-posterior commissure by using a pulsed-gradient, spin-echo, single-shot echo planar imaging method (TR=8 seconds, TE=101 msec; 128×128 matrix, field of view=24 cm). This analysis used slices that began 5 mm below the anterior commissure and proceeded superiorly because this part of the brain encompassed the largest extent of cerebral white matter.
One hundred twenty-four contiguous coronal images (slice thickness=1.5 mm) were acquired through the whole head by using a three-dimensional fast spoiled-gradient recall acquisition sequence with IR-Prep (TR=12.7 or 14.7 seconds, TE=4.5 or 5.5 msec, field of view=22 cm) in a 256×256 matrix. In addition, an oblique axial dual double-echo spin-echo scan (TR=5 seconds, TE=22/90 msec, 256×256 matrix, field of view=240 mm, 26 slices, 5-mm slice thickness, 0-mm gap) was acquired at the same slice positions as the diffusion tensor images, providing a pair of T2- and proton density-weighted volumes. The T2/proton density volumes were used to correct image distortion on the diffusion tensor images and for image segmentation.
Fractional anisotropy maps
(21,
22) for each subject were computed and registered to Talairach space
(23) by applying three image registration steps. First, all spoiled-gradient recall acquisition volumes were matched to a target volume in Talairach space by using an in-house nonlinear registration software program
(24,
25). This algorithm is based on previously published methods
(26–
28) with additional features for computational efficiency
(24,
25). Second, the T
2- and proton density-weighted volumes were registered to the spoiled-gradient recall acquisition volume within each subject
(29). Third, the b=0 diffusion tensor imaging volume was registered to the T
2 volume to correct for spatial distortion in fractional anisotropy maps
(24,
25). The transformations from these three registration steps were combined numerically and applied to the fractional anisotropy maps by using a single interpolation operation.
A white matter mask was created for each subject with FSL software
(30) by segmenting the brain on the basis of the T
2, proton density, and spoiled-gradient recall acquisition volumes. The white matter mask was also transformed to Talairach space. The transformed white matter masks were averaged and thresholded at 40% to obtain a white matter mask for each group. Both the registered fractional anisotropy and white matter images were smoothed with a three-dimensional isotropic Gaussian kernel with a sigma of 3 mm.
Group differences in demographic variables were examined by using independent-group t tests or chi-square tests. To control for potential partial volume effects in our analysis, fractional anisotropy values for each voxel were adjusted for white matter intensity through linear regression. Two-sample t tests comparing patients and healthy subjects were performed at each voxel on the corrected fractional anisotropy values. Voxels that had a t value greater than 3.85 (p<0.001, two-tailed) and were part of a spatially contiguous cluster size of 100 voxels or greater were considered to be significantly different between groups.
Results
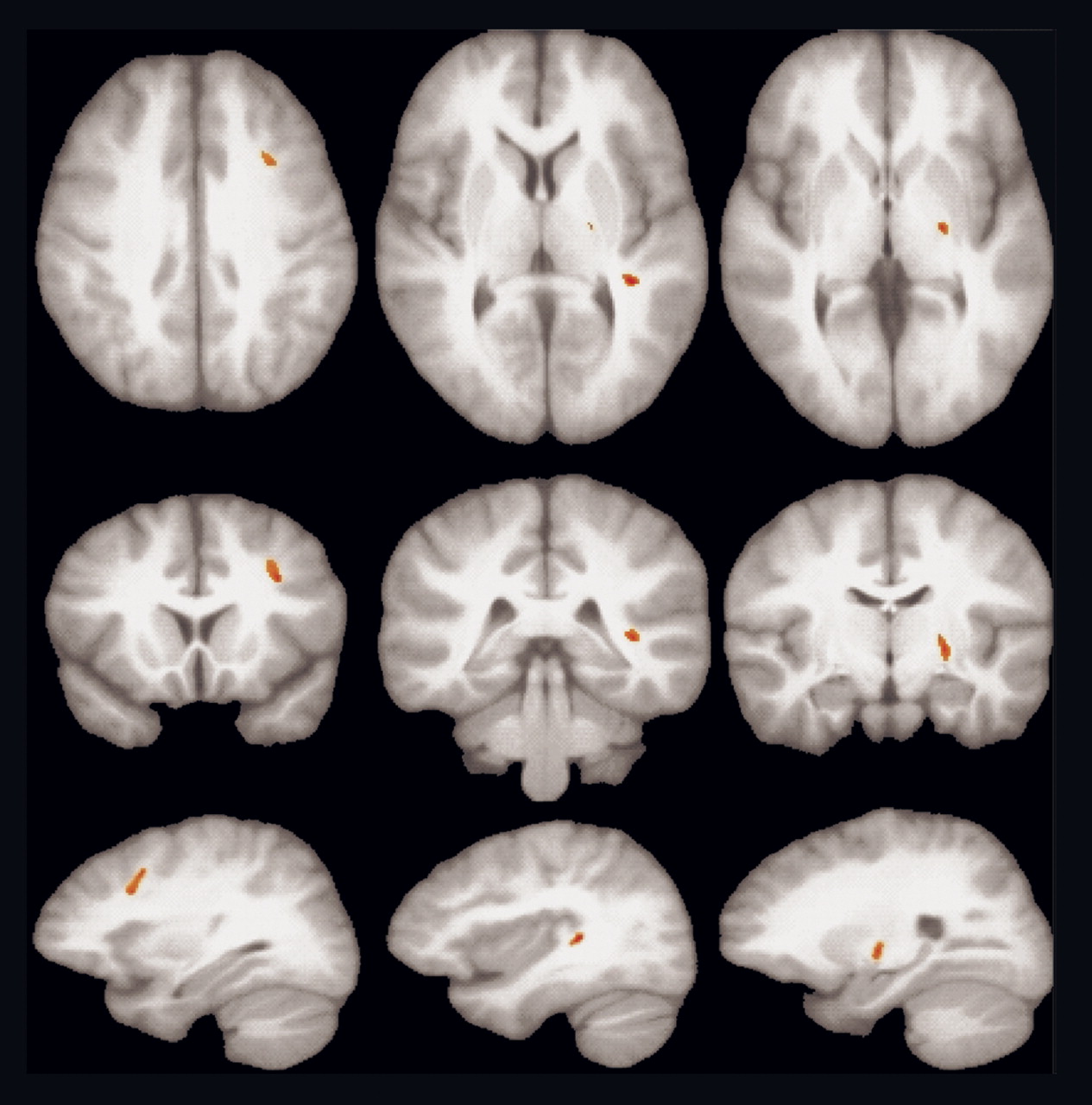
Patients and comparison subjects did not differ significantly in distribution of age, sex, parental social class, race, or handedness (p>0.05). Significantly lower fractional anisotropy in patients compared with healthy volunteers was observed in three regions of the left hemisphere (
Figure 1), including the white matter in the middle frontal gyrus (t=5.27, df=20, p<0.001, cluster size=225), in the posterior superior temporal gyrus (t=5.08, df=20, p<0.001, cluster size=106), and in the internal capsule extending into the globus pallidus (t=4.76, df=20, p<0.001, cluster size=129). Talairach coordinates representing the centroid (x=right, y=anterior, and z=superior) in the middle frontal gyrus were x=–30, y=15, z=35; in the temporal lobe they were x=–36, y=–32, z=8; in the internal capsule/globus pallidus they were x=–22, y=–12, z=2. Similar findings were obtained when analyses were restricted to the subgroup of patients who had never taken antipsychotic drugs. There were no areas of significantly higher fractional anisotropy in patients compared with healthy volunteers.
Discussion
Our findings suggest that patients experiencing a first episode of schizophrenia or schizoaffective disorder exhibit lower anisotropic diffusion in brain white matter. Lower anisotropic diffusion could be associated with either micro- or macrostructural alterations involving the myelin sheath and/or directional coherence of fiber tracts. Abnormalities appeared less pronounced in this group of patients experiencing their first episode of schizophrenia or schizoaffective disorder than has been found in studies of patients with chronic illness
(8,
9). In that regard, our findings converge with those of Bagary et al.
(31), who reported relatively circumscribed white matter abnormalities inferred from magnetization transfer imaging in first-episode patients compared with healthy volunteers.
Previous diffusion tensor imaging studies that quantified anisotropic diffusion in patients reported abnormalities in the prefrontal white matter
(11,
12) and corpus callosum
(5,
6) as well as more widespread abnormalities in the brain
(8,
9). Other diffusion tensor imaging studies specifically investigating white matter connectivity reported abnormalities in the uncinate fasciculus in patients compared with healthy volunteers
(7,
10). Some studies, however, did not identify group differences in anisotropy
(13,
14). Differences in illness duration, image processing, and analysis, including the use of region-of-interest versus voxel-wise approaches, could account for discrepant findings.
Decreased fractional anisotropy was observed among patients in the left middle frontal gyrus white matter, which is consistent with postmortem findings
(4,
32). Moreover, this part of the middle frontal gyrus corresponds anatomically to the dorsolateral prefrontal cortex, which has also been widely implicated in the pathophysiology of schizophrenia
(33). We also observed lower fractional anisotropy among patients in the white matter in the vicinity of the left planum temporale and Heschl gyrus. Our findings thus converge with those of previous volumetric magnetic resonance imaging studies implicating less left-hemisphere gray matter in these regions among patients experiencing their first illness episode
(34) compared with healthy volunteers.
There are several limitations to this study, including the small number of subjects and risk of a type I error. To limit the possibility of a type I error, however, we investigated fractional anisotropy only in the brain white matter; therefore, it appears noteworthy that we did not observe any areas of increased fractional anisotropy in patients, thus strengthening the specificity of the observed findings. A possible limitation of the segmentation is that it may have reduced contrast of the lentiform nucleus with the surrounding white matter, thus increasing the risk of tissue misclassification in the area of the globus pallidus.
In summary, we present evidence for white matter pathology as inferred from diffusion tensor imaging in patients with schizophrenia or schizoaffective disorder examined early in the course of illness. Future studies could use diffusion tensor imaging to examine whether such abnormalities are progressive as well as possible sex differences in white matter microstructure in schizophrenia
(35).