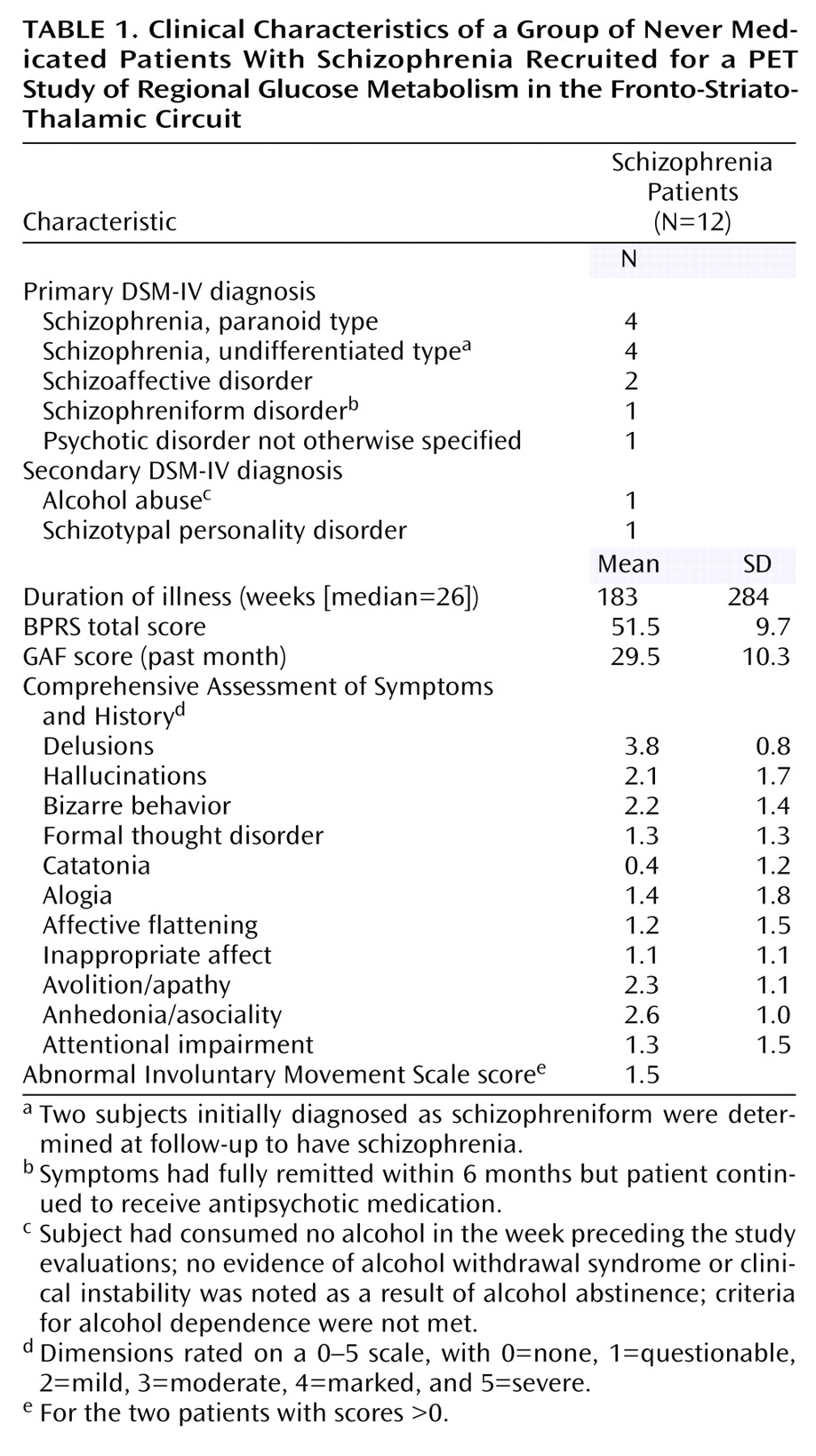
The prefrontal cortex, striatum, and thalamus form a neural circuit important in regulating sensory input, attention, and action. Deficits in these three areas in schizophrenia have been widely reported in both structural
(1) and functional
(2) brain imaging studies. The thalamus comprises multiple nuclei that relay and filter sensory and higher-order inputs to and from the cerebral cortex and limbic structures. Thus, it is a candidate structure for abnormality in schizophrenia, a disease that includes disturbed sensory and attentional function
(3). Especially important within the thalamus may be the medial dorsal nucleus (with its interconnections with the prefrontal cortex) and the pulvinar (also interconnected with frontal and temporal regions). Both nuclei interact with the cortex in high-level cognitive activity and have been found to have reduced volumes in schizophrenia in postmortem as well as magnetic resonance imaging (MRI) studies
(4–
6).
In our earliest positron emission tomography (PET) studies, which used
18F-fluorodeoxyglucose (FDG) and employed older-generation PET scanners with lesser in-plane resolutions of 15 mm
(7) or 7.5 mm
(8), we did not find decreased metabolic rates in the thalamus of unmedicated (previously medicated) schizophrenia patients compared with healthy subjects. In a study of never medicated schizophrenia subjects, we found less prominent regional glucose metabolic rate (rGMR) in the mediodorsal nucleus region
(6). In another study of never medicated patients
(9), blood flow decreases in the thalamus, frontal lobe, and temporal lobe were identified. With increased spatial PET resolution and coregistered structural MRI (1.2-mm thick slices) scans obtained for the entire thalamic volume, lower rGMR in the medial dorsal nucleus of unmedicated schizophrenia patients relative to healthy subjects was statistically confirmed, although whole thalamus metabolism did not differ between groups
(10). However, when the nuclei were traced on coregistered MRI templates, decreased metabolic rates in the medial dorsal and centromedian nuclei
(11), but not the pulvinar, were confirmed. Decreased rGMR in the remainder of the thalamus was again not found, indicating specificity of the effect to the medial association regions. That study, while large (61 normal subjects and 40 patients), was limited in having patients who, although unmedicated, had previously been treated with neuroleptics, which might have affected thalamic volume and rGMR. In addition, the uptake condition was the serial verbal learning task, which activates the frontal lobe but is not known to activate the pulvinar.
Results
SPM Mapping
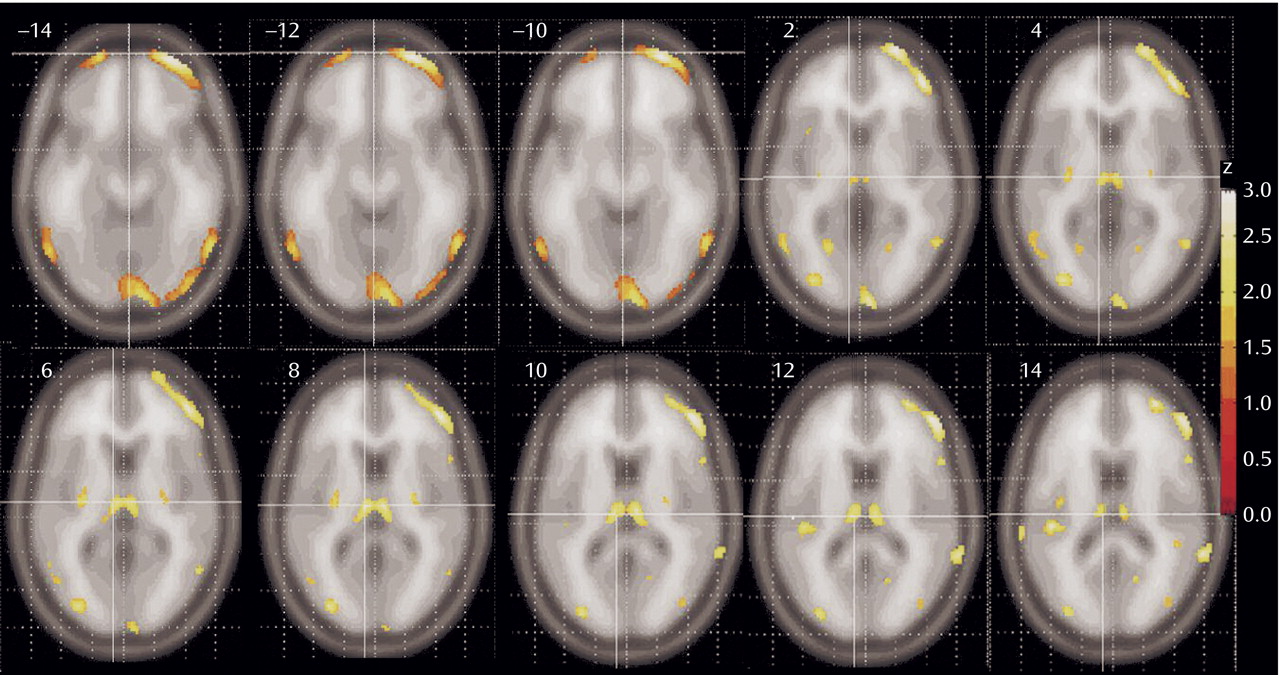
Patients with schizophrenia had lower relative metabolic rates in the thalamus and lateral prefrontal cortex, especially at the orbitofrontal levels (
Figure 2). A significant reduction in metabolic rate was also noted in additional cortical areas, including the insula, temporal lobes, parietal lobe (Brodmann’s area 39), and the anterior cingulate at its most dorsal level (Brodmann’s area 24)
(22). The posterior putamen was also less active in the schizophrenia subjects. Small regions in the temporal poles and medial temporal lobe are less prominently represented than the prefrontal cortex among areas significantly lower in patients with schizophrenia. No gray matter areas were found significantly higher in patients with schizophrenia than healthy comparison subjects.
Pixel-by-Pixel Examination of the Thalamus
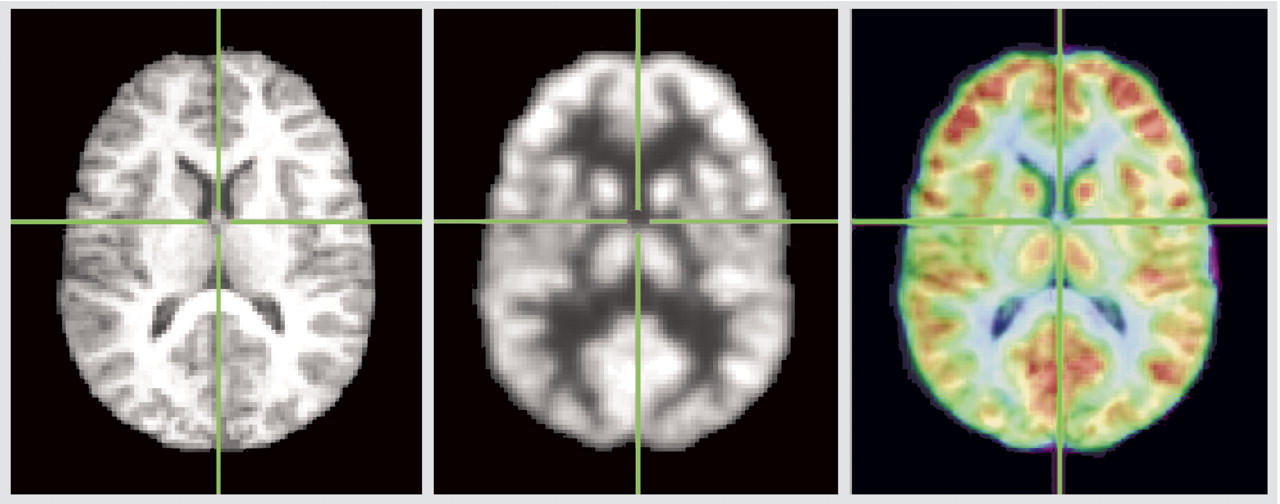
Coregistration is illustrated in
Figure 3, and detailed examination of Talairach z=12 slice in
Figure 4. The red area in
Figure 4 indicates pixels where the healthy subjects have higher relative metabolic rates than patients with schizophrenia (p<0.05, two-tailed), which extends across the region of the medial dorsal nucleus and pulvinar.
Stereotaxic Region-of-Interest Examination of the Thalamus
The medial dorsal nucleus had a relative metabolic rate significantly higher in healthy volunteers (mean=11.8, SD=0.64) than patients with schizophrenia (mean=11.2, SD=0.64) (t=2.88, df=23, p=0.008). The whole thalamus did not differ between normal subjects and patients (mean=10.0 [SD=0.43] and 9.7 [SD=0.49], respectively). Repeated measures two-way analysis of variance (ANOVA) with whole thalamus and medial dorsal nucleus for the right and left hemisphere revealed a significant diagnostic group difference (F=6.66, df=1, 23, p<0.02) and a significant interaction between group and region of interest (whole thalamus, medial dorsal nucleus alone)(F=6.45, df=1, 23, p<0.02), indicating that the metabolic group difference effect for the medial dorsal nucleus was significantly greater than for the whole thalamus. There was no significant diagnostic group-by-hemisphere or diagnostic group-by-hemisphere-by-structure interaction.
MRI Template-Traced Nuclei
A lower metabolic rate in the medial dorsal nucleus for patients with schizophrenia (mean=2.59, SD=0.29) versus healthy subjects (mean=2.76, SD=0.28) was confirmed (F=4.62, df=1, 18, p<0.05), and this was specific to the medial dorsal region when compared with the remainder of the thalamus in a three-way ANOVA (diagnostic group [healthy, schizophrenia] by structure [medial dorsal, remainder of thalamus] by hemisphere [right, left]), with a significant group-by-structure interaction (F=5.51, df=1, 18, p<0.04). It is of interest that all subjects except one schizophrenia patient had higher relative values in the medial dorsal nucleus than in the remainder of the thalamus. While the relative metabolic rate was lower in the pulvinar in patients with schizophrenia, this difference did not reach statistical significance. The correlation between the stereotaxic medial dorsal nucleus and the traced medial dorsal nucleus was 0.51.
Task Performance
All healthy subjects and all but one patient performed the task above chance levels and completed responses in every trial block during the uptake period. Removal of the one schizophrenia subject who did not perform the task did not alter the significance of the group comparison t test on the medial dorsal nucleus (t=2.31, df=22, p=0.03) or the diagnostic group difference with ANOVA (F=4.32, df=1, 22, p<0.05).
Discussion
Our results confirm earlier studies that reported relative metabolic rate reduction in the thalamus and lateral prefrontal cortex in patients with schizophrenia, extending those findings in several important ways. We have demonstrated these findings in a group of never medicated patients, indicating that these reductions could not be due to medication artifacts. Further, the use of an uptake condition demonstrated to have thalamic activation in earlier PET studies enhances the strength of the conclusions. Replicating the results with methodologically complementary pixel-by-pixel t test methods and traced templates allows both whole-brain exploration and the specific regions to be exhaustively tested. These specific methodological steps appear to have enhanced the power of the FDG PET images to confirm group differences in comparison with some earlier studies that used medicated patients, an eyes-closed resting condition, or lower-resolution image acquisition.
The limitations of our present study include the relatively modest number of never medicated subjects, image resolution and coregistration quality, and the use of relative metabolic rates. While somewhat smaller than two earlier studies of never medicated patients
(6,
9), we had adequate power to detect differences in thalamic rGMR. Methods are currently being explored to overcome image resolution limitations by applying a partial volume correction to the image data. However, this correction has not been fully validated and has the potential to introduce a large bias into the analysis
(23,
24). With a structure as small as the thalamus or medial dorsal nucleus (6–9 mm wide), image coregistration and the quality of structure position standardization are critical. We evaluated joint error in our examination of FDG image coordinates and found that about 75% of subjects’ FDG landmarks adjacent to the medial dorsal nucleus lay within one 2-mm pixel of a standard location and of the matching MRI template. Last, we thresholded the significance probability images at the p<0.05 level without correction. This lacks correction for multiple t testing, but since the study was focused specifically on the medial dorsal region (the relative metabolic rate of which was previously reported to be reduced
[11]), and the dorsolateral prefrontal cortex was previously explored with significance probability mapping
(25), it seemed appropriate to consider the maps as confirmatory rather than exploratory. The medial dorsal region selected a priori and assessed using stereotaxic position also yielded a p=0.0083 probability (0.0041 one-tailed in replication). While absolute metabolic rates for the medial dorsal nucleus could have been obtained with the FDG method, there is no evidence that total brain metabolic rate differs between normal subjects and patients with schizophrenia. Thus the small regions of thalamic and prefrontal metabolism reductions seen in
Figure 2 seem unlikely to have artifactually arisen on the basis of other large areas of cortical metabolic rate shifts.
One might argue that patients with schizophrenia lacked motivation to perform the task and that differences in the medial dorsal nucleus were an indirect artifact of diminished involvement in successful task performance, rather than an indication of primary regional pathology. Against this is the fact that all but one patient participated in the task and performed well above chance levels (results were unchanged after omission of the one nonperforming patient). Further, motivational areas (Brodmann’s areas 11, 12) and vigilance areas (Brodmann’s area 32) did not show significant decrease or increase, suggesting that failure in nonspecific task engagement regions is not entirely responsible for the medial dorsal nucleus deficit. It is interesting that patients with obsessive-compulsive disorder may have higher blood flow in their thalamus
(26). Thus, potential reciprocal interactions between spatial attention (medial dorsal nucleus) and motivation (orbital and medial prefrontal cortex), the impairment of which is a central feature of the schizophrenia syndrome, cannot be ruled out.
Our use of unmedicated and previously untreated patients is important in relating the findings to schizophrenia rather than medication effects. Studies have reported decreases in relative metabolic rate in the frontal lobe with clozapine
(27) and haloperidol
(28) but not with risperidone
(29), so the use of unmedicated patients is especially important for establishing this finding. It is interesting that patients with higher dorsolateral prefrontal metabolic rates were reported likely to respond to clozapine
(30).
Other studies have also confirmed reduced thalamic FDG uptake in schizophrenia
(31,
32) (reviewed elsewhere
[11]). Last, using functional MRI (fMRI), reduced activation in the thalamus has been observed. The anterior thalamic region as well as the medial and anterior frontal lobe had reduced fMRI blood oxygenation level-dependent activation in medicated patients with schizophrenia during the continuous performance test of vigilance
(33), both areas adjacent to but not exactly overlapping with our regions. Less thalamic activation in schizophrenia was also seen in other fMRI studies
(34). However, significant thalamic activation was observed in medicated patients with schizophrenia but not in normal subjects during performance of the Sternberg memory task
(35), although apparently no direct map of group differences in activation was presented.
Examination of the pixel-by-pixel maps indicates that medial and posterior portions of the thalamus appear to be the main regions of the thalamus with decreased metabolic rates. The thalamus comprises multiple nuclei that relay and filter sensory and higher-order inputs to and from the cerebral cortex and limbic structures
(3). The biggest thalamic substructures, the medial dorsal nucleus and the pulvinar (both visible on MRI), are of particular interest in attention because of their reciprocal connections with the prefrontal and temporal regions and because their size and metabolic rate appear diminished in imaging studies of patients with schizophrenia. The medial dorsal nucleus has strong interconnections with the dorsolateral prefrontal cortex
(36), and the connections of the medial dorsal nucleus have been used to define the prefrontal cortex
(37), a key area of executive action and focusing of attention thought to be defective in schizophrenia (reviewed by Buchsbaum and Hazlett
[2]). Crosson
(38) suggests the medial dorsal nucleus as a critical element in an attentional “selective engagement” system that impacts semantic functions in schizophrenia.
It should be noted that the use of never medicated patients may be especially important in establishing regional metabolic differences in the thalamus and that even thalamic volume may be affected by neuroleptics
(39). The recent report of decreased thalamic D
2 receptor binding in the medial portion of the thalamus in drug-naive patients with schizophrenia
(40) indicates that both chronic and acute medication effects could influence thalamic metabolic rate. That important study not only supports a role for the medial thalamus in schizophrenia but also suggests that the medial regions of the thalamus are potentially informative for understanding medication effects as well.
Until the mid-1970s it was thought that the prefrontal cortex received its thalamic input solely from the medial dorsal nucleus; however, it is now apparent that the pulvinar also contributes to its innervation
(41,
42). The pulvinar, important in visual and possibly auditory attention
(43–
45), also has prominent interconnections with the parietal and temporal lobe. The current findings of decreased metabolic rate in the medial and posterior regions of the thalamus, prefrontal cortex, and posterior temporal and parietal regions tend to confirm a network of frontothalamic and parieto-temporal-thalamic rGMR concurrently diminished in patients carrying out a task demonstrated to activate the pulvinar
(12).
Much work in the field of visual neurophysiology suggests that the pulvinar signals the importance or relevance of stimuli that fall inside classically defined visual receptive fields
(43). Neurophysiological studies in animals have found that neurons of the pulvinar respond specifically to stimuli to be the target of a saccade
(46). These findings suggest that the pulvinar may play a role in “visual salience,” that is, the attention-related selection of a target
(44), and “directed attention”
(12). The anatomy and projections of the pulvinar suggest functional cortical-thalamic-cortical loops involved in a variety of functions including salience, attention, and working memory
(43). The fact that both the medial dorsal nucleus and the pulvinar express D
2 type dopamine receptors
(40), together with their demonstrated role in selective attention, fits well with recent formulations of schizophrenia as a hyperdopaminergic state that leads to an aberrant assignment of salience to elements of one’s experience
(47). According to that model, both typical and atypical antipsychotics “dampen the salience” of abnormal experiences via blockade of D
2 receptors and by doing so, allow the resolution of symptoms.