All psychiatrists are familiar with antipsychotic, antianxiety, and antidepressant medications, whose origins date to the 1950s. We who have been in the field since the early days of psychoactive medications will recall that there were real questions in the 1960s about whether these medications were specific treatments or just sedating agents. More recently, we have come to rely on medications that are effective against bipolar disorder, phobias, posttraumatic stress disorder, and obsessive-compulsive disorder. Although most psychiatrists would now agree on the specificity of these medications, it took time and multiple randomized, controlled trials for clinicians to become convinced.
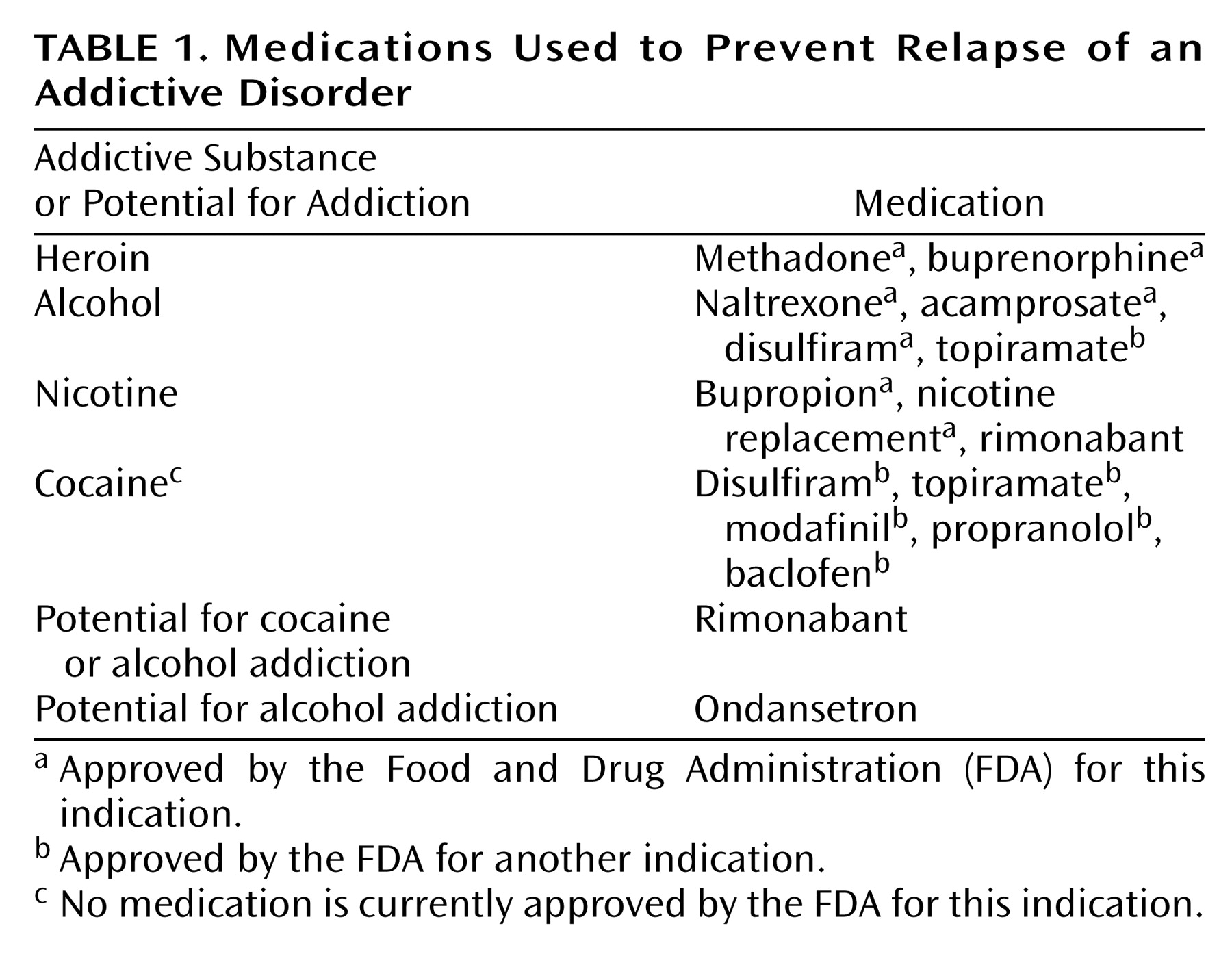
Over the past 25 years, we have gradually developed a list of medications that have been found in randomized, controlled trials to be effective in the treatment of addictive disorders. The immediate goal is the reduction of drug craving and the prevention of relapse to compulsive drug taking. The number of such medications is increasing, with an additional one (acamprosate) approved by the Food and Drug Administration (FDA) in 2004 joining buprenorphine, which became available to clinicians in 2002. Still, many psychiatrists are not aware that medications are effective in the treatment of addictive disorders. Some still subscribe to the adage, “You can’t treat a drug problem with a drug.”
Detoxification of drug-dependent patients is noncontroversial, time-limited, and easy to understand. The patient is simply given a medication in the same category as the drug of dependence, and withdrawal symptoms are blocked. Then the medication can be gradually reduced at a rate determined by the half-life of the drug of dependence. Evidence has not been presented to show that long-term outcome after very rapid detoxification under general anesthesia is more effective than detoxification over days
(1). Thus, opiate withdrawal symptoms can be treated by methadone or buprenorphine, and nicotine withdrawal can be treated with a nicotine patch. Another approach to opiate withdrawal is to block brain pathways involved in producing the symptoms with a medication such as clonidine or lofexidine
(2). Lofexidine is not yet available in the United States, but it is widely used in the United Kingdom as a nonopiate medication for opiate withdrawal.
Naltrexone as a Model Anticraving Medication
A good example of an anticraving medication is naltrexone for the treatment of alcoholism. Naltrexone was developed for the treatment of opiate addiction and was only applied to alcoholics because of animal studies showing that alcohol activated the endogenous opioid system
(15). It was tested in a series of open trials in the 1980s and subsequently in a randomized, controlled trial in the alcohol program of the Philadelphia Veterans Administration Medical Center
(12,
16). After replication at another site
(17), the FDA added alcoholism to the indications for naltrexone. It is interesting to note that both of these first two studies measured craving for alcohol, and it was lower in the group randomly assigned to naltrexone (who also drank less alcohol).
In recent years, animal models of addiction relapse have been developed. The animal is allowed to establish consistent drug-taking behavior and then is withdrawn from the drug. After a period of abstinence, it is tested for reinstatement (relapse) of drug taking. Relapse test procedures fall into three categories: stress, conditioned cues, and drug priming. Stress is modeled by small amounts of foot shock that provoke reinstatement of drug taking. Conditioned cues are stimuli (sound, light, and odor) that previously have been paired with drug availability, and drug priming involves giving a small dose of the drug the animal was taking. With these procedures, the animal quickly reinstates the prior level of response to the drug. Medications can be tested in these animal models, and so far, they are proving to be fairly predictive of the response in patients.
Naltrexone is an example of a medication that consistently reduces alcohol consumption in animals. It appears to do so by blocking opiate receptors that modulate the release of dopamine in the reward system, specifically, the nucleus accumbens
(18). Alcohol alone increases extracellular dopamine in the nucleus accumbens, but in animals pretreated with naltrexone, the alcohol-induced dopamine increase is blocked. Further evidence of the role of the endogenous opioid system in alcohol reward is that mu receptor knockout mice do not self-administer alcohol
(19).
Dopamine has not been measured directly in humans given alcohol, but there are studies that are consistent with the hypothesis that alcohol releases endogenous opioids that are involved in the activation of neurotransmitters, such as dopamine, resulting in the rewarding effects of alcohol. Gianoulakis et al.
(20) reported that test doses of alcohol produce significantly larger increases in plasma beta endorphin in nonalcoholic volunteers with a family history of alcoholism than in volunteers with no alcoholism in their family backgrounds. Although this is a pituitary response and is not necessarily reflective of central endogenous opioids, a parallel CNS effect is suggested by reports of naltrexone blockade of the stimulation or a “high” produced by alcohol in volunteers with a family history of alcoholism. Volunteers with no family history of alcoholism did not report stimulation from alcohol
(21). Furthermore, during randomized, controlled trials, patients randomly assigned to naltrexone reported that when they slipped and drank alcohol, the expected high was blocked
(22,
23). Thus, both animal data and clinical studies support the hypothesis that the endogenous opioid system is involved in the subjective effects of alcohol.
The animal models of relapse indicate that naltrexone blocks cue-induced relapse but is less effective against stress-induced relapse
(24). In human alcoholics, most studies have examined the effects of naltrexone or placebo in combination with some form of counseling or psychotherapy in the prevention of relapse to clinically significant drinking. In these studies, relapse could be related to stress, cues, the priming effect of a small dose of alcohol, or other nonspecified factors. A large majority of randomized, controlled trials found that patients who were randomly assigned to naltrexone had significantly fewer relapses than the placebo groups, but the reasons for relapses are usually not known. One study, however, examined the effects of naltrexone on cues for alcohol drinking and found a reduction in the urge to drink in response to alcohol cues
(25) and improved outcome at 1 year
(26).
Craving is based on subjective reports, and no animal model has yet been developed to study this human phenomenon. There is evidence, however, that the dopamine system can be conditioned so that cues previously associated with drug availability can activate the reward system. This has been reported in humans by using brain imaging
(27,
28) and in animals by using microdialysis. An example of the latter is the work of Gonzales and Weiss
(18), who demonstrated an increase in dopamine levels in the nucleus accumbens as soon as an animal was placed in a chamber where it
expected to have access to alcohol. Naltrexone did not block this conditioned dopamine increase, but it did block the pharmacological increase in dopamine produced by alcohol. In humans during randomized, controlled trials, naltrexone was reported to suppress craving compared to alcoholics given placebo in several studies where craving was measured (e.g., references
12,
17,
29).
One of the most elegant models for studying the effects of a priming dose of alcohol on craving in humans was developed by O’Malley and colleagues
(30). Nontreatment-seeking alcoholics who were pretreated with naltrexone or placebo were given a single priming drink of alcohol in the laboratory. Those receiving naltrexone had lower craving levels at baseline, and craving did not significantly change after alcohol priming. The placebo-treated patients reported higher craving at baseline, and craving was significantly increased by the priming drink. The reports of craving correlated well with drinking behavior because alcohol was made available to the participants as part of the experiment. Participants had the choice of more alcoholic drinks or money instead of each drink after the priming drink. The placebo group chose to drink significantly more than the naltrexone group, resulting in a progressively increasing blood alcohol level, whereas the naltrexone group reported less craving during alcohol availability, consumed fewer drinks, and drank them more slowly when they did drink. This human laboratory study was remarkable in that it produced results consistent with both animal models and clinical trials. A human laboratory model for stress-induced relapse has also been reported but has not yet been used to test medications
(31).
A major problem with all outpatient treatments of long duration, whatever the diagnosis, is variability of adherence to a medication regimen. Obviously, a medication cannot help if it is not taken regularly. Naltrexone, despite a plasma half-life of only 10–12 hours (including its active metabolite), has activity at mu opiate receptors in the human brain for over 48 hours
(32; unpublished 2004 paper by M.E. McCaul et al.). However, activity at delta receptors is less and more variable, and the duration of activity at kappa opiate receptors is unknown. Activity at all three types of receptors may be essential for full clinical benefit
(33), and thus, a daily dose of naltrexone is recommended. Several studies have shown efficacy in preventing relapse only if patients not taking naltrexone regularly are excluded
(34,
35). Despite heterogeneity among alcoholics and problems of variable medication adherence, the number of randomized, controlled trials finding naltrexone effective in alcoholics greatly outnumbers the negative trials. To address the problem of adherence, a major advance in the clinical usefulness of naltrexone is currently underway with the introduction of depot formulations. These new formulations provide active blood levels for 30–40 days after a single injection
(10,
11). FDA approval of one of these formulations is expected within the next 2 years and will greatly facilitate the treatment of both alcoholism and heroin addiction.
Heredity has been found to play a major role in all forms of drug addictions. Although alcoholism has received the most attention, nicotine, heroin, and cocaine all have been shown to be influenced by heredity
(36). Genes clearly influence the pharmacological effects of alcohol. The well-known alcohol-flushing reaction has been shown to be mediated by the genes for both alcohol dehydrogenase and aldehyde dehydrogenase
(37), and the risk of developing alcoholism varies as expected, according to the severity of the genetically determined flushing after alcohol ingestion. Heredity also influences the behavioral sensitivity to alcohol. A low response to alcohol (tolerance) was found to be more common in the sons of alcoholics, and this tolerance predicted a fourfold increase in the risk of developing alcoholism within 10 years
(38). As described, volunteers with a family history of alcoholism also showed a greater beta endorphin response to alcohol
(20) and a greater stimulation effect from alcohol
(21). In the laboratory study by King et al.
(21), naltrexone was found to block stimulation in the volunteers with a family history of alcoholism. These human laboratory findings are consistent with animal laboratory data and data from clinical trials.
Heterogeneity of Response
If we consider the evidence for a genetic component to addictive disorders, it is not surprising that there are a wide variety of responses to anticraving medications. Among alcoholics, there appears to be a subgroup that responds to alcohol with an increase in plasma beta endorphin and probably a similar increase in CNS endogenous opioids that can be neutralized by an opiate receptor antagonist. Although there is no practical way to identify this subcategory at present, analyses of randomized, controlled trials have provided some help to clinicians. Thus, a strongly positive family history of alcoholism points to a poor prognosis for a placebo group but a good response to treatment with naltrexone
(39,
40). Similarly, the subgroup of alcoholics that reported a high level of alcohol craving did poorly when they were randomly assigned to placebo but achieved a good response to naltrexone therapy
(40). Previous typologies of alcoholism focused on age at onset and clinical characteristics
(41–
43) have not been studied with respect to naltrexone response.
Obviously, greater precision is desirable in the selection of patients for naltrexone treatment. One potential advance would be to identify alcoholics likely to respond to treatment involving the blockade of opiate receptors. The activation of the endogenous opioid system by alcohol could vary according to the opioid peptides released
(44) and according to the sensitivity of the opiate receptors. A number of polymorphisms have been identified for the gene encoding the mu opiate receptor, and one of them, the Asp40 variant, has been found to have high affinity for beta endorphin in vitro
(45) and altered function during in vivo studies
(46,
47). Alcoholics with this variant who were enrolled in randomized, controlled trials had a poor outcome when they were randomly assigned to placebo but a good outcome when they received naltrexone
(48). Of course, there is evidence from animal studies that the blockade of delta and kappa opiate receptors, in addition to mu receptors, is involved in the effects of naltrexone on alcohol drinking
(33). Nevertheless, the finding of a genotype that predicts success with naltrexone, if replicated, will provide a pharmacogenetic tool to enhance the matching of patients to treatment and encourage the search for additional functional polymorphisms.