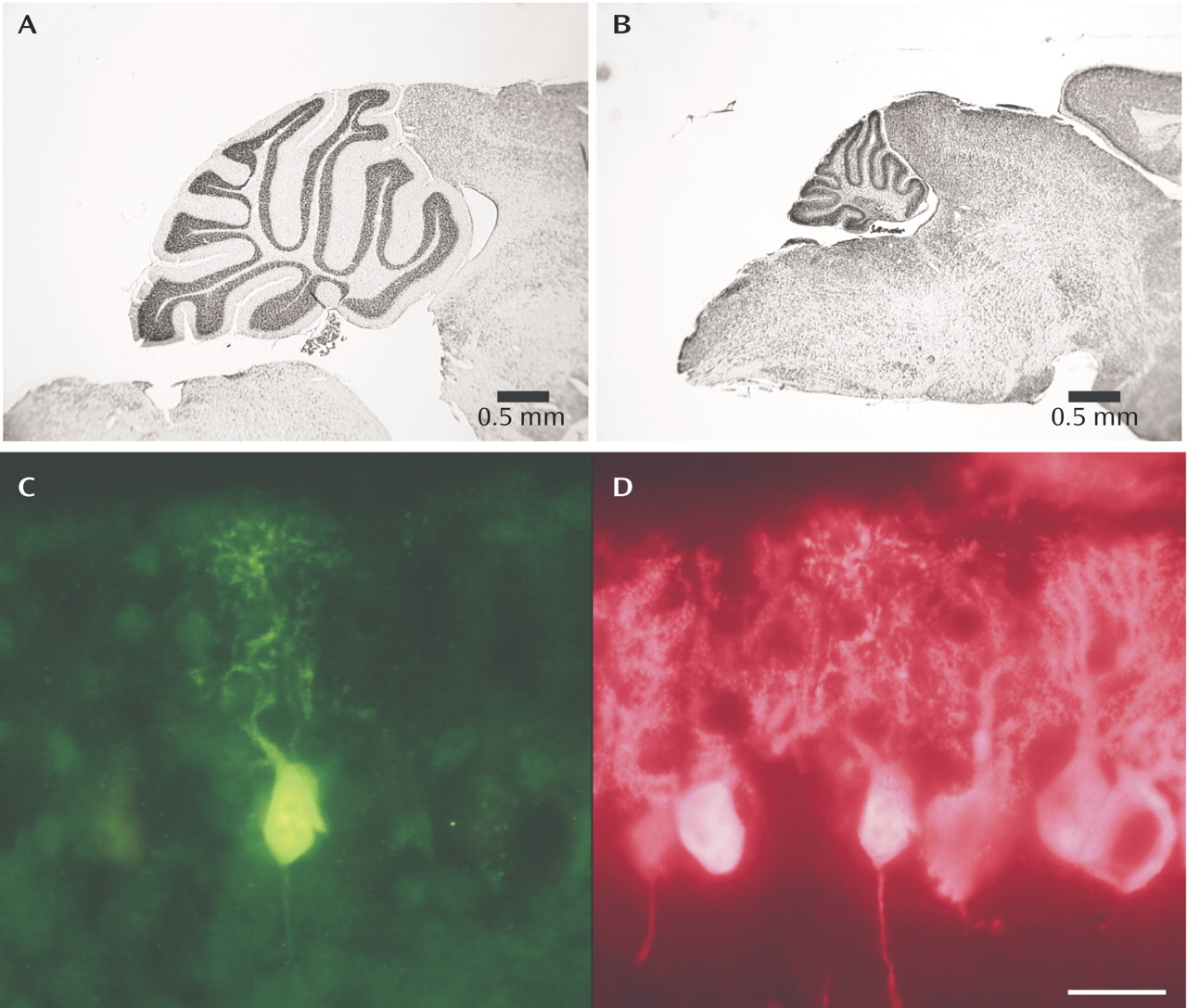
The top
figures illustrate the magnitude of volume loss in the Lurcher cerebellum (B) relative to normal mouse cerebellum (A) due to the caspase-mediated apotosis that occurs in the Lurcher mouse Purkinje cells. In (C), a dying neuron is shown in bright green expressing significant amounts of activated caspase-3, one of the cell death proteins. In the same section, a Purkinje cell-specific calcium binding protein labels the healthy neurons (bright pink) but not the dying neuron (D).
During human development, approximately half of the neurons that are originally laid down in the brain are estimated to die during the process of central nervous system formation. Many neurodegenerative diseases, including Huntington’s, Alzheimer’s, and Parkinson’s, are thought to involve cell death, a process called apoptosis. Moreover, the regulation of cell death continues to be an important physiological process throughout the lifespan of an animal as a mechanism to respond to disease and injury. It is not surprising that a complex cellular machinery has evolved to regulate cell death during development and after injury. Many of the genes and protein families involved in cell death and survival have been identified. One animal model involving regulated cell death is a mouse mutant called Lurcher; this model has been used to identify many of the molecular events involved in cell death. The Lurcher mutation is a single base-pair substitution in the gene for the delta-2 glutamate receptor (one of the glutamate receptor subtypes in the cerebellum) that aberrantly codes for a receptor with a continuously open sodium channel instead of a channel that opens and closes in responsive to local modulation. This delta-2 glutamate receptor is predominately expressed in cerebellar Purkinje cells; the Lurcher Purkinje cells start to die after the first week of postnatal development. Virtually all of the Purkinje cells and many of the neurons that connect to the Purkinje cells die within a month of birth (see Figure), greatly reducing the size of the cerebellum.
The mechanisms of cell death in the Lurcher Purkinje cells are thought to model for some of the mechanisms involved in human neurodegenerative disorders like Huntington’s, Alzheimer’s, and Parkinson’s. Several important key molecules involved in the regulation of cell death belong to a family called Bcl-2 proteins. There are “good” Bcl-2 proteins: e.g., Bcl-2, Bcl-xL, Bcl-w, and Boo; these promote cell survival. And there are “bad” Bcl-2 proteins: e.g., Bax, Bak, and Bim; these promote cell death. Indeed, the ratio of Bcl-2 to Bax within a cell is thought to modulate the ultimate outcome of the cell. If there is more Bcl-2 then Bax, the cell will live; if more Bax than Bcl-2, then the cell will die. The cell death pathway is initiated by the activation of another class of proteins (suitably named “executioner” proteins) called caspases that are already present in most cells in an inactive form. A high ratio of Bax to Bcl-2 activates caspases family members to obliterate cellular proteins and DNA, leading to cell death. The Lurcher Purkinje cell in the Figure (bright green cell) is immunolabeled for activated caspase-3, one of the final caspases in the cell death pathway, indicating a cell slated for death. This dying Purkinje cell is surrounded by other Lurcher Purkinje cells that have not yet begun to die (bright pink cells). These same proteins (among others) regulate cell death in humans during development, life and death. Moreover, this same caspase-mediated cell death pathway can be initiated by any number of physiological stressors, including loss of trophic support, oxidative stress, drugs, and ionizing radiation. The Lurcher mouse is used in the study of these cell death pathways, informing mechanisms of neurodegeneration in humans.