Although bipolar disorder is characterized by a loss of emotional homeostasis, particularly the development of mania, cognitive symptoms represent a significant part of this illness. Bearden et al.
(1) highlighted that bipolar patients exhibit attentional, memory, and executive impairments that are worse during acute affective episodes but persist during euthymia. The association of cognitive abnormalities with mood episodes is not surprising, given the reciprocal connection between brain networks that modulate mood and cognitive functions
(2). However, the persistence of cognitive deficits in the absence of mood symptoms, i.e., during euthymia, suggests that these symptoms may reflect the underlying dysfunctional neurophysiology of bipolar disorder more directly, independent of mood state.
During euthymia, bipolar patients exhibit minimal symptoms by definition, although a persistent vulnerability for mood dysregulation is always present. This persistent vulnerability has been hypothesized to result from overreactive emotional (i.e., anterior limbic) brain networks
(3–
5), which may be reflected in cognitive disturbances (i.e., through reciprocal interactions between cognitive and mood networks) even during euthymia. Consistent with this hypothesis, we recently reported evidence of anterior limbic overactivation during a simple attentional task in euthymic, unmedicated bipolar patients
(4). Although patients with euthymic bipolar disorder in this study performed the task similarly to healthy subjects, they exhibited activation in different cortical regions, perhaps to compensate for interference from emotional brain networks in order to maintain task performance. A next step in this research direction is to incorporate a more challenging attentional task in which compensatory mechanisms might be overwhelmed in patients but not healthy subjects, thereby further differentiating brain activation patterns between these groups.
One approach toward developing a more difficult attentional task is to incorporate cognitive interference. Cognitive interference refers to the introduction of a secondary stimulus that interferes with the processing of the primary stimulus. In the classic Stroop interference task, subjects are presented names of colors (e.g., “red,” “blue”) written in colored ink and are asked to identify the color of the ink. In the original description, Stroop found that subjects were slower at identifying the color of the ink when it was discordant with the word (e.g., the word “red” written in blue ink) than when simply identifying colored squares
(6). This and similar tasks are widely used in cognitive research, and bipolar patients often exhibit decreased Stroop task performance compared with healthy subjects
(1). The classic color Stroop task is difficult to study using functional magnetic resonance imaging (fMRI) since it requires verbal responses, which introduce movement that interferes with image acquisition. To address this problem, Bush et al.
(7) developed a counting Stroop interference task that can be integrated within the fMRI environment. The validity of this interference task was supported by 1) delayed responses in the interference versus control condition in healthy subjects and 2) the expected activation in anterior cingulate and dorsolateral prefrontal cortex consistent with radioimaging studies of other Stroop interference tasks.
Discussion
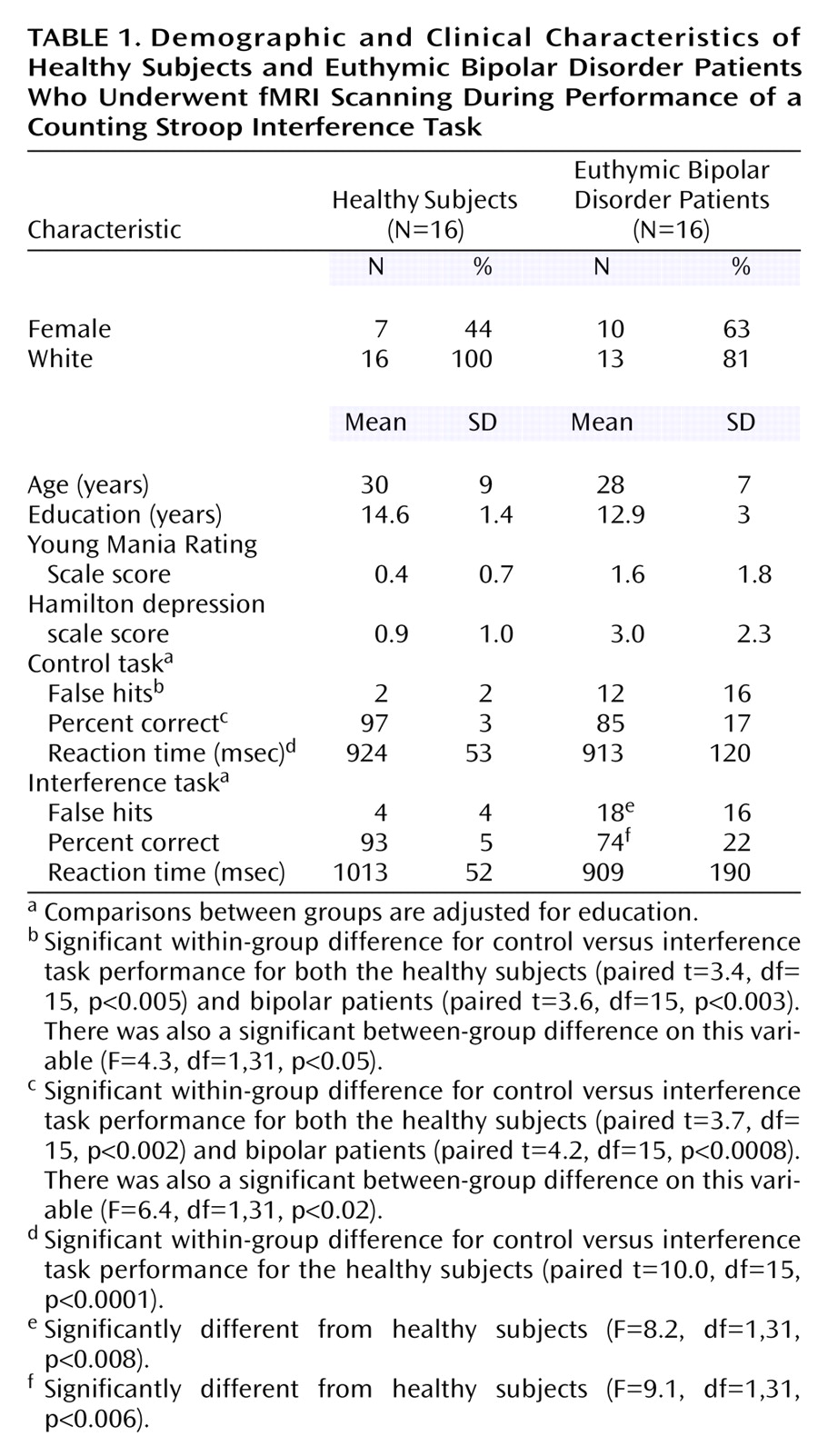
As predicted, the bipolar patients exhibited significantly poorer performance than the healthy subjects on the counting Stroop interference task, which persisted even after we controlled for an unexpected poorer performance on the control task. The pattern of impairments in the bipolar subjects suggests an impulsive response bias that was associated with more incorrect responses (false hits) and a failure to slow down during the interference condition in order to improve performance (as healthy subjects did). Consequently, this tendency in the patients toward impulsive responding may be reflected in brain activation differences.
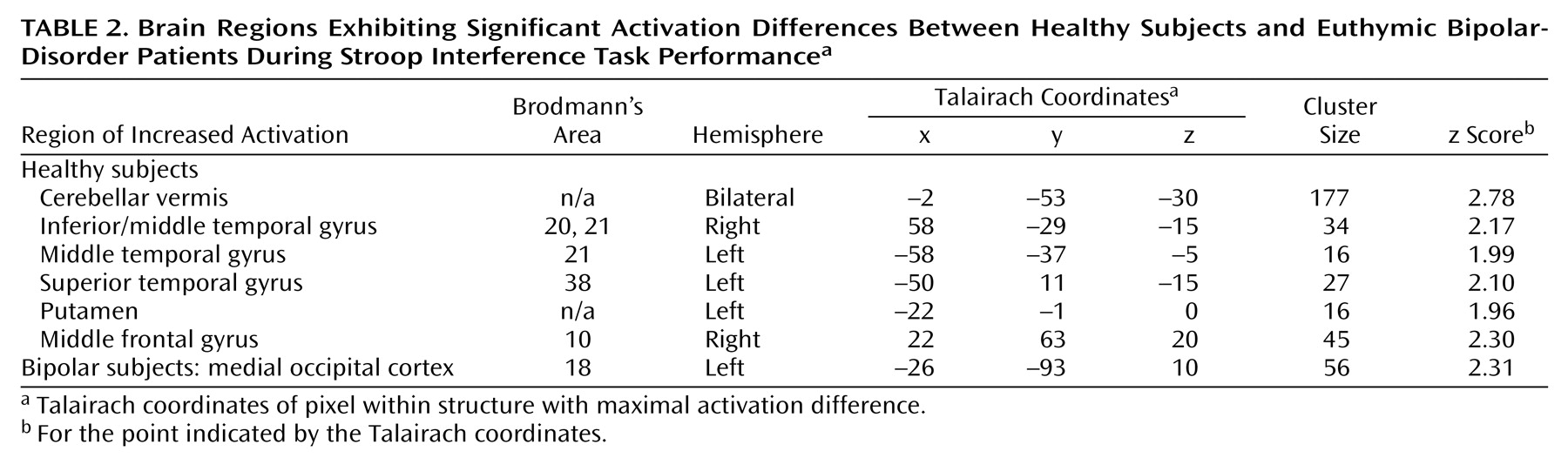
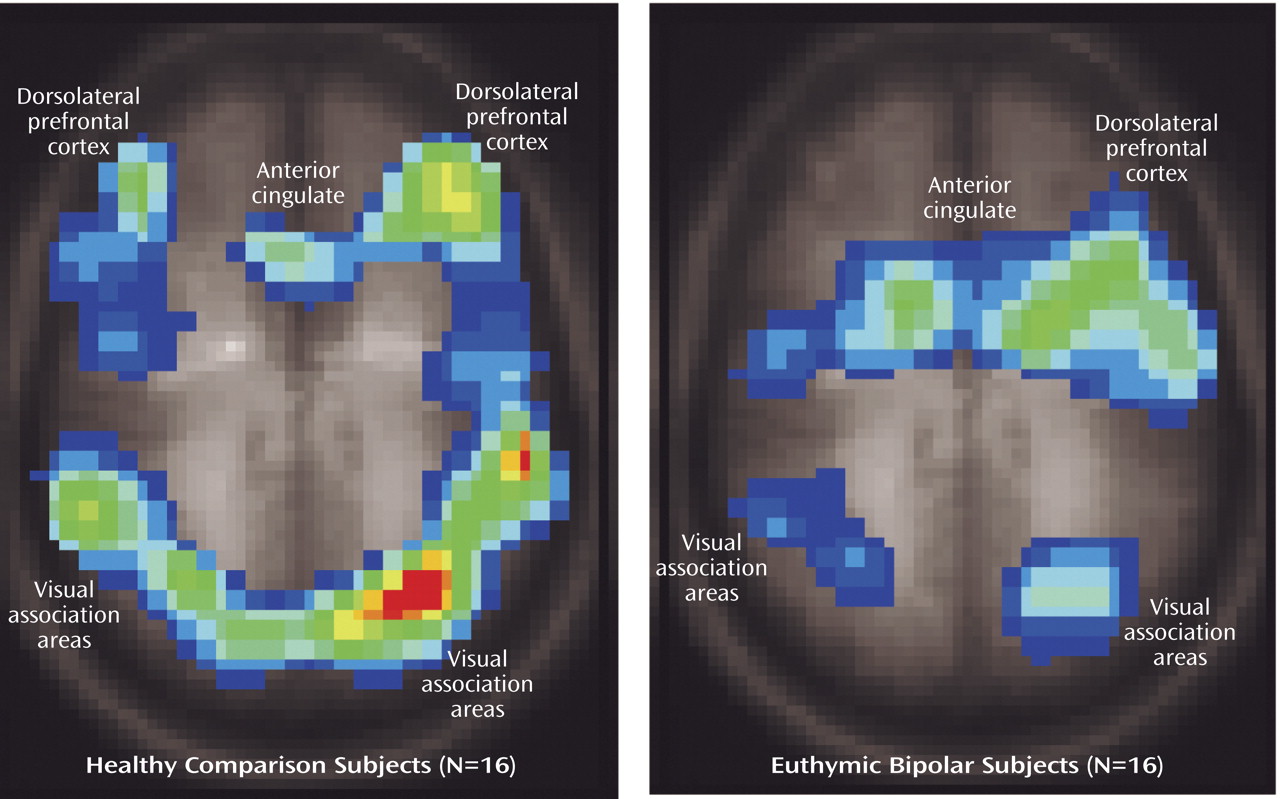
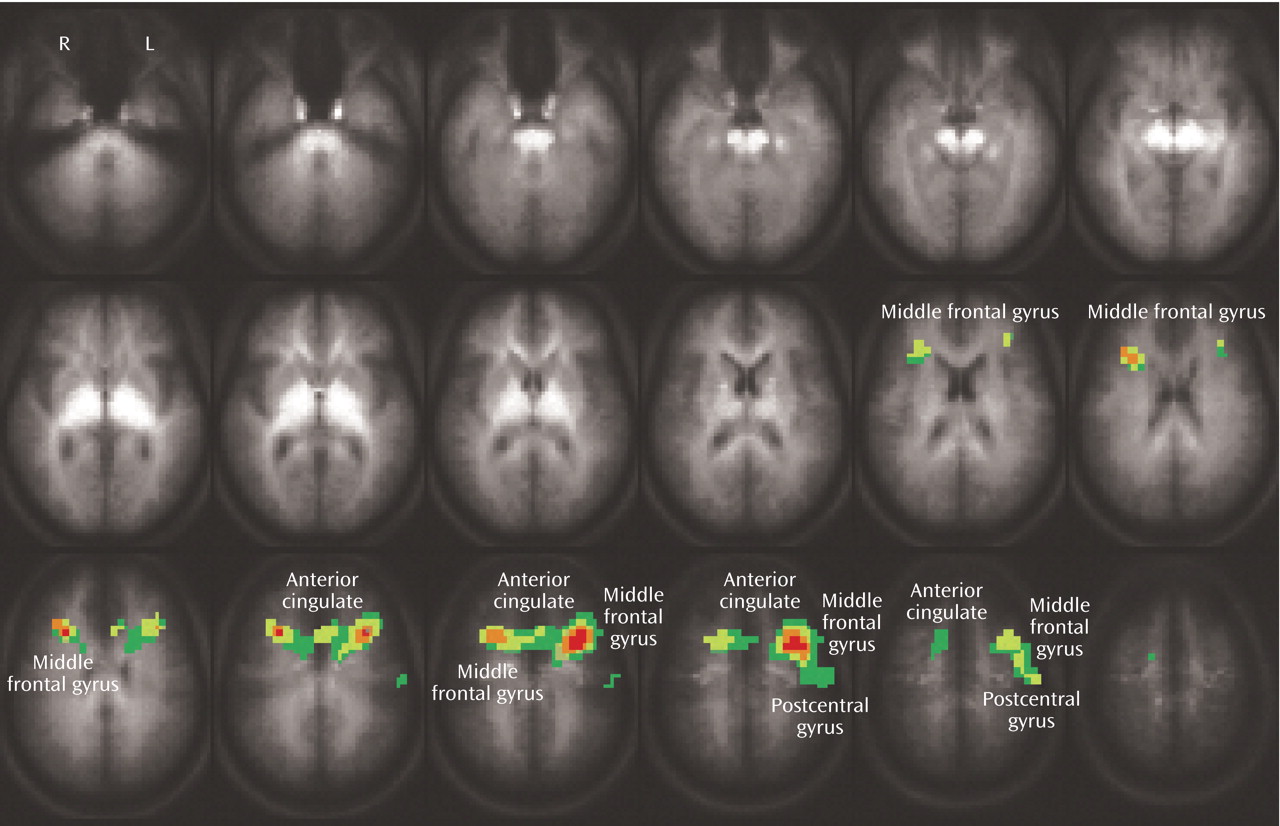
Both the healthy and bipolar subjects exhibited activation of the anterior cingulate (Brodmann’s area 24/32) and dorsolateral prefrontal brain regions (Brodmann’s area 9) while performing the Stroop task. As noted previously, these regions have been shown to consistently activate during interference tasks
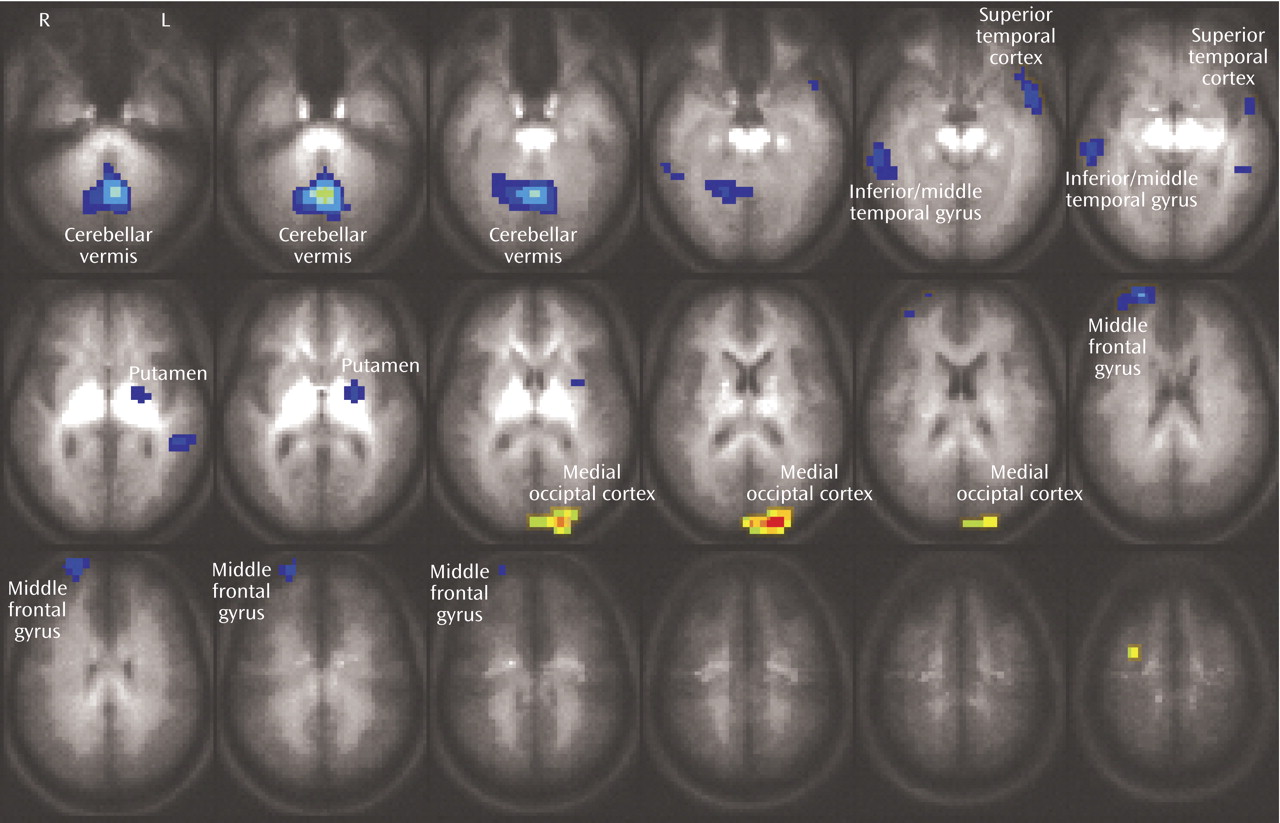
(7), and activation in these regions did not significantly differ between groups. Instead, the bipolar patients demonstrated a pattern of less activation in temporal regions, midline cerebellum, ventrolateral prefrontal cortex, and putamen, suggesting failure to activate secondary brain regions involved in this task. Since bipolar patients did not slow their responses in the more demanding interference condition, i.e., they did not exhibit the same speed-accuracy tradeoff as the healthy subjects, it suggests that activation of these brain areas may contribute to effective task performance, perhaps by modulating impulse control.
Activation of the midline cerebellum, striatum, and superior temporal gyrus has been associated with error detection and response inhibition
(17–
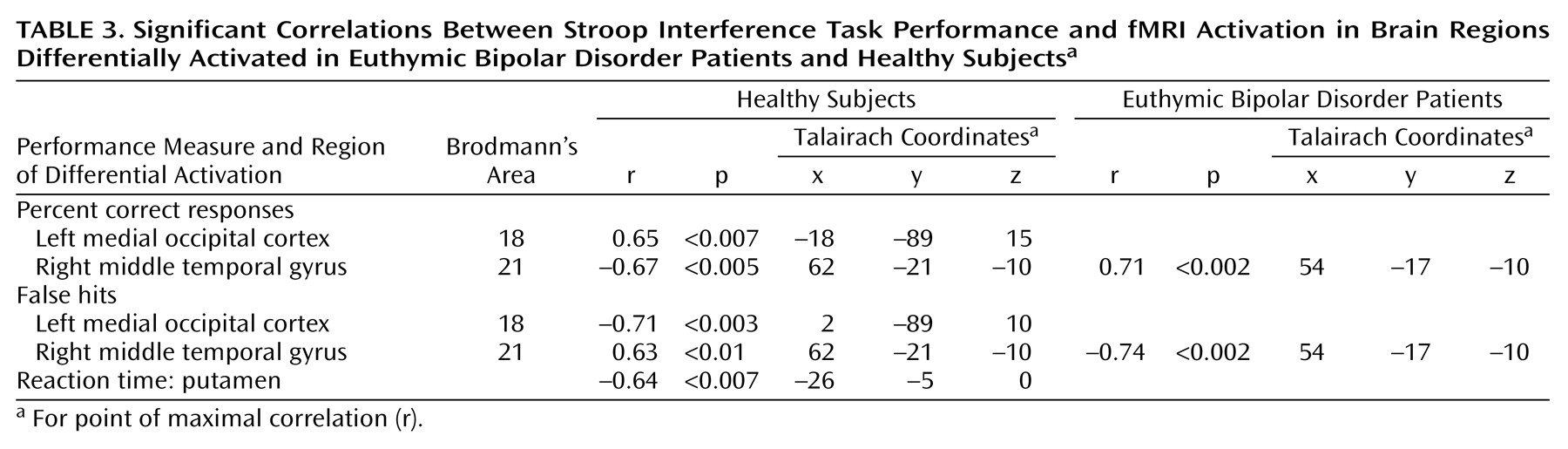
20). In the current study, putamen activation was inversely correlated with reaction time in healthy subjects (but not bipolar patients), supporting its role in these functions. Moreover, in healthy subjects, activation in the right middle temporal gyrus was inversely correlated with the percentage of correct responses, and directly correlated with incorrect responses, suggesting that this region, too, may have been involved in error detection and correction. In contrast, the bipolar patients demonstrated the opposite correlations in the temporal cortex, and no significant correlations in the occipital cortex or putamen, suggesting failure to appropriately activate these brain areas to compensate for interference. Additionally, activation in the ventrolateral prefrontal cortex (Brodmann’s area 10) has been associated with decision making and resolving conflict
(21), and it appears to be recruited as cognitive tasks become more difficult
(22). Similar to our finding in this study, previous investigators identified decreased activation in the right ventrolateral prefrontal cortex during a decision-making task in mania
(23) and blunted activation in the homologous left hemisphere region across mood states in bipolar patients during a Stroop task
(9). In addition, previous neuroimaging studies have identified structural, functional, and neurochemical abnormalities in many of these same brain regions, including the superior temporal gyrus
(24,
25), midline cerebellum
(26–
28), and striatum
(29,
30). Together, the activation differences and associations with task performance observed in this study suggest that the bipolar patients underactivated brain regions that are involved in error detection, response inhibition, and conflict resolution, thereby leading to poorer task performance and a more impulsive response pattern, as evidenced by the lack of a speed-accuracy tradeoff. The specific reasons why this network of error detection/response inhibition brain regions was underactivated in these patients is not clear. However, our previous work suggests that abnormalities within primary mood networks in bipolar disorder, even during dysthymia, may disrupt cognitivenetworks
(4) because of reciprocal activation of these systems. The underactivation in this error detection/response inhibition network may reflect this disruption. Alternatively, bipolar patients may have primary dysfunction within this network. Additional imaging studies that integrate emotional, cognitive, and impulse control paradigms would be useful to examine these possibilities.
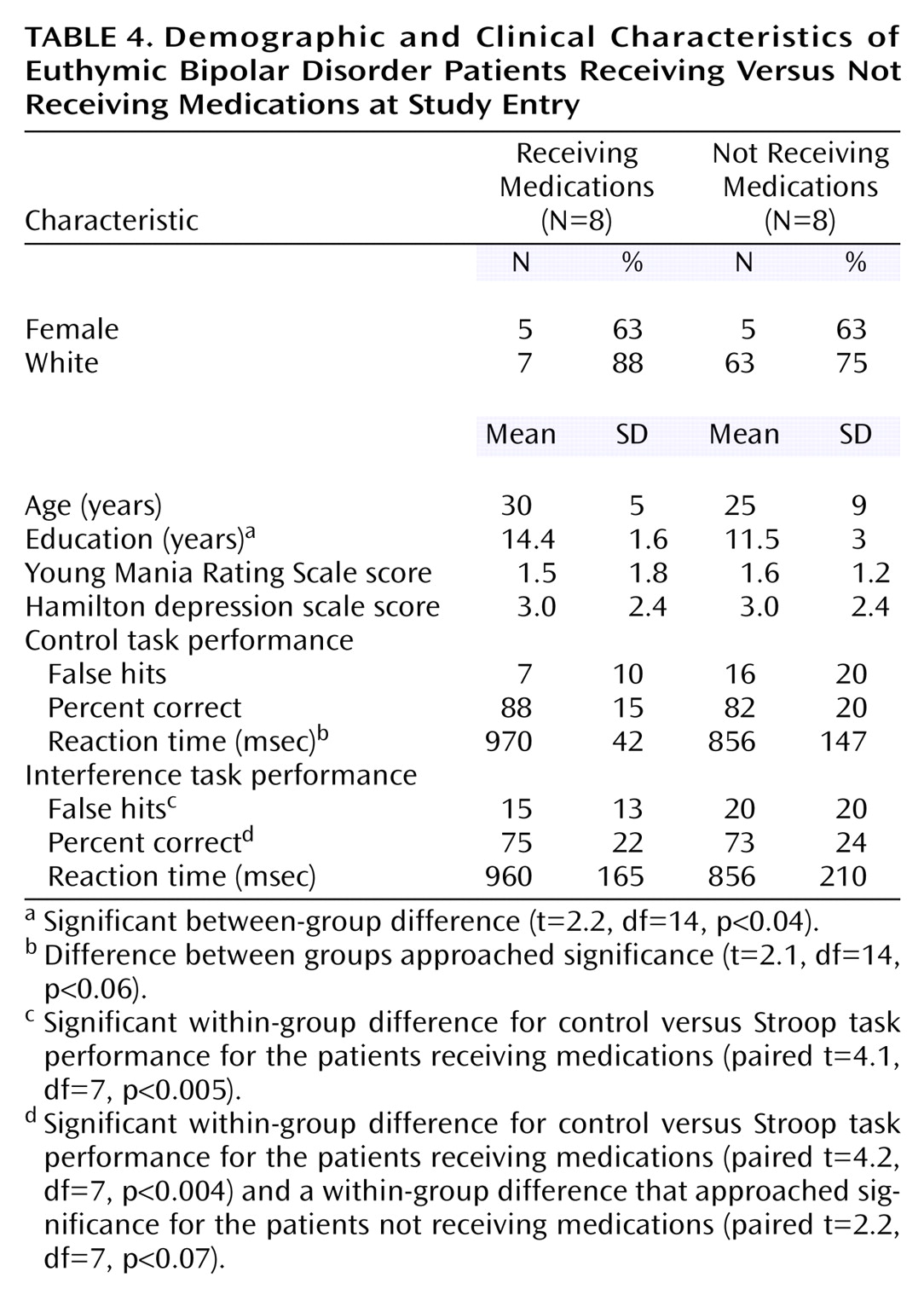
Since none of the brain regions differentially activated between bipolar and healthy subjects were also differentially activated between patients who were versus those who were not receiving medication, it minimizes the likelihood that the group differences resulted from medication exposure in the bipolar subjects. Instead, medication use was associated with activation in the dorsolateral prefrontal cortex and anterior cingulate. One interpretation of this finding is that medications help to restore function in these brain regions primarily involved in interference tasks, leading to increased activation. Consistent with this suggestion, the patients receiving medications exhibited numerical, although not significant, improvement in task performance. Specifically, the effect of medication appeared to slow and decrease variability in reaction time in the control task so that response patterns were more similar to healthy subjects in the medicated than the unmedicated groups. However, the small number of subjects and multiple medications administered limit comparisons between medicated and unmedicated patients. This analysis should be considered exploratory and the results primarily speculative; the main utility of this analysis is to aid in interpreting this particular confound for the larger (i.e., healthy versus bipolar) comparison.
Several other limitations must be considered when interpreting this study. First, the overall number of subjects is relatively small, such that only large effects were identified. This limitation is particularly true for subgroup comparisons (i.e., patients receiving and not receiving medications), as noted. Nonetheless, this study informs a number of hypotheses for future work with larger subject numbers. Second, the patients receiving medications were being treated with a variety of drug combinations, so that specific medication effects could not be determined with the number of subjects available. The results suggest, however, that medications may alter brain activation in regions that are associated with cognitive tasks so that studies of specific drug effects on cognition (and the corresponding brain activation) in larger subject numbers are warranted. Third, the patients exhibited poorer performance on the control task, which makes interpretation of the study more difficult than if they had exhibited incrementally worse performance only on the interference task. Nonetheless, even after we controlled for the control task differences, differences in interference task performance persisted. Finally, we did not assess for the presence of a history of attention deficit hyperactivity disorder (ADHD) in this group of bipolar patients. ADHD comorbidity is relatively common in bipolar disorder and might contribute to impulsive responding. However, these patients were well known to us from their participation in the outcome study, and no one exhibited obvious symptoms of ADHD, but in the absence of a formal assessment this cannot be certain. Offsetting these limitations are several study strengths including 1) a well-defined, truly euthymic bipolar patient group; 2) measurement of task performance in the scanner to ensure that the task was being attended to and to permit examination of associations between brain activation and task performance; and 3) a specific approach toward examining medication effects.
In summary, during a counting Stroop interference task, patients with euthymic bipolar disorder exhibited significant activation differences from healthy subjects. These differences appeared to occur predominantly in brain regions that activate during error detection, response inhibition, and conflict resolution, suggesting that the bipolar subjects failed to appropriately activate brain areas to prevent impulsive responding.