The fusiform gyrus, located on the ventromedial surface of the temporal and occipital lobes, has been demonstrated to be a key brain region for face perception
(4–
6). The findings of both neurophysiological and functional magnetic resonance imaging (fMRI) studies have suggested that faces are perceived, at least in part, by a processing stream separate from that used in processing other objects
(7). Data from studies of event-related potentials have demonstrated that the negative potential recorded at occipitotemporal leads, the N170 potential, is larger in response to faces than to objects in healthy subjects
(8,
9). On the basis of converging evidence from electroencephalography
(6) and magnetoencephalography
(9), the fusiform gyrus has been considered to be one of the main neural sources of the N170 potential in response to human faces. The N170 potential is considered to be an index of aspects of face analysis
(8). Liu et al.
(10) have suggested that the N170 potential recorded with magnetoencephalography is associated with extraction of the identity of individual faces. However, there is no uniform agreement that the fusiform gyrus is the main neural source of the N170 potential; for example, the superior temporal sulcus region has been also reported to be the site of N170 generation
(11). One of the goals of the present study was to determine whether anatomical abnormalitites of the fusiform gyrus revealed by MRI are associated with N170 abnormality. Data confirming this association would strengthen the evidence for a link between the fusiform gyrus and the N170 potential.
Despite extensive research on face recognition in healthy subjects and the potential relevance of face processing in social interactions, few functional and anatomical studies have examined face processing in schizophrenia. Existing studies have focused primarily on facial affect recognition
(12,
13). However, very few studies have examined the functional integrity of early processes related to face sensory features. There is also a paucity of data on the relationship between functional indices of face processing and structural correlates. Previously we reported an association between structural abnormalities of the fusiform gyrus and behavioral measures of memory function for faces in schizophrenia
(14). These results provided the impetus for the present study, which focuses on linking electrophysiological and structural correlates of face processing in schizophrenia.
The present study was designed to test the hypotheses that patients with schizophrenia, compared with healthy subjects, show less activation in early visual processing for faces, as measured by the N170 potential in response to faces, and that this functional abnormality is associated with smaller fusiform gyrus volume in schizophrenia patients, compared with healthy subjects. Confirmation of these hypotheses would further clarify the contributions of the fusiform gyrus to the N170 potential.
Results
Demographic Characteristics and Behavioral Performance
No significant group differences were found in age, handedness, or parental socioeconomic status. The patients had significantly lower socioeconomic status than the healthy comparison subjects (t=–3.84, df=34, p=0.001), consistent with deficits in functioning due to the disorder. No significant group differences were found in total intracranial contents (t=1.23, df=27, p=0.23), accuracy of target responses (patients: 98.7%; comparison subjects: 99.4%) (t=1.02, df=34, p=0.32), or median reaction time (patients: median=498 msec; comparison subjects: median=478 msec) (t=–0.75, df=34, p=0.46).
N170 Amplitude
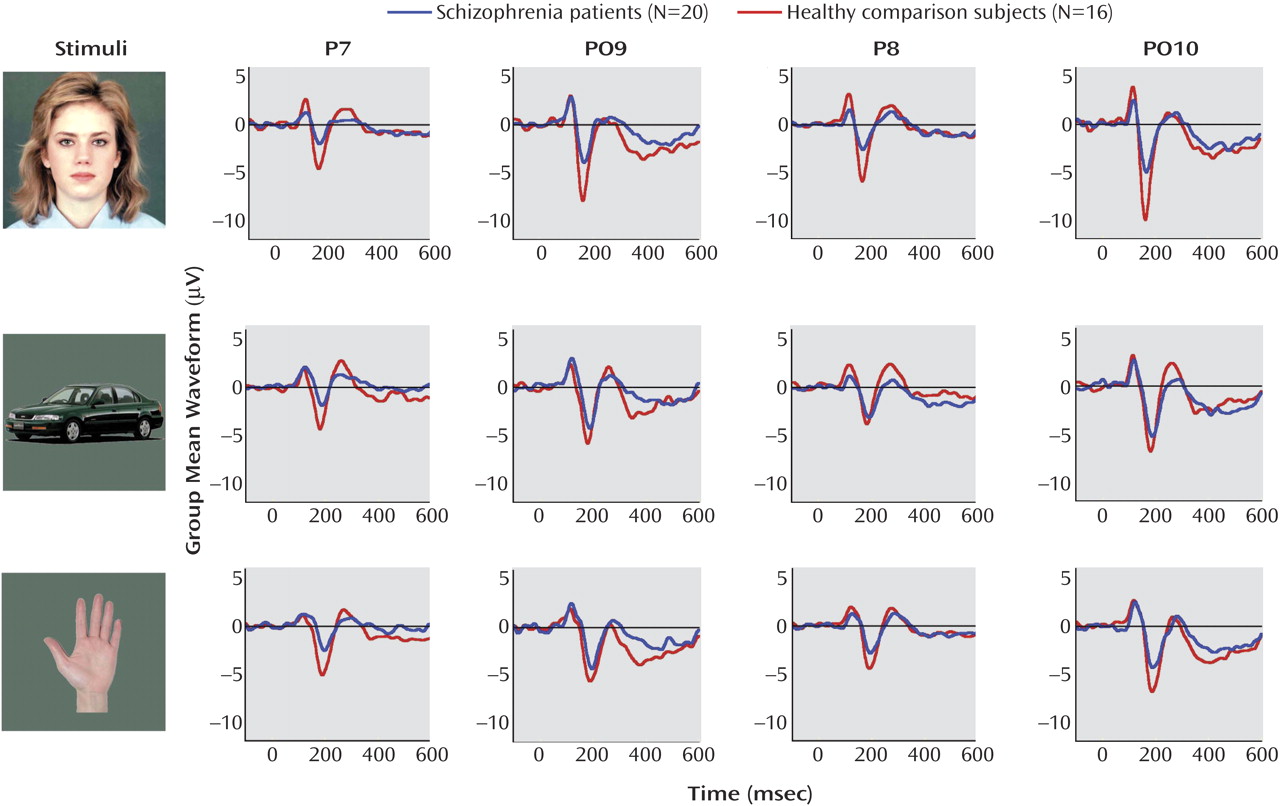
For N170 amplitude (illustrated in
Figure 2), a repeated-measures ANOVA demonstrated a significant main effect of stimulus (F=4.46, df=2, 33, p=0.02) and electrode site (F=16.03, df=2, 33, p<0.001) but no main effect of hemisphere (F=1.33, df=1, 34, p=0.26) or group (F=3.79, df=1, 34, p=0.06). There was a significant stimulus-by-group interaction (F=4.92, df=2, 33, p=0.02). To further delineate this result, Helmert’s contrasts were performed on the stimulus-by-group interaction. The groups differed in average N170 amplitude in response to images of faces, compared to the average amplitude in response to images of hands and cars (F=9.79, df=1, 34, p=0.004), but they did not differ in the average amplitude in response to images of hands, compared to the average amplitude in response to images of cars (F=2.45, df=1, 34, p=0.13). Thus, the group difference in N170 amplitude was specific to faces. In addition, the N170 amplitude for each stimulus was analyzed by using two-factor ANOVAs, with electrode site (three sites) as a within-subjects factor and group as a between-subjects factor. In these follow-up analyses, the schizophrenia patients showed significant N170 amplitude reduction in response to faces, compared to the healthy comparison subjects (F=6.94, df=1, 34, p=0.01), as well as a reduction in response to images of hands (F=3.41, df=1, 34, p=0.07), but not to images of cars (F=0.75, df=1, 34, p=0.39). Finally, to examine the stimulus effect in each group, N170 amplitude was analyzed using two-factor ANOVAs with stimulus (images of faces, cars, or hands) and electrode site (three sites) as within-subjects factors. The healthy comparison subjects showed a significant main effect of stimulus (F=6.24, df=2, 14, p=0.01), with the most negative N170 amplitude recorded in response to images of faces (group mean: –7.83 μV), relative to images of hands (group mean: –6.44 μV) and cars (group mean: –5.66 μV). The schizophrenia patients did not show this effect (F=0.16, df=2, 18, p=0.86), indicating that their N170 amplitude was not moderated in response to the different stimuli. For the patients, the N170 amplitude was –4.6 μV in response to images of faces, –4.5 μV in response to images of hands, and –4.7 μV in response to images of cars.
Fusiform Gyrus Volume
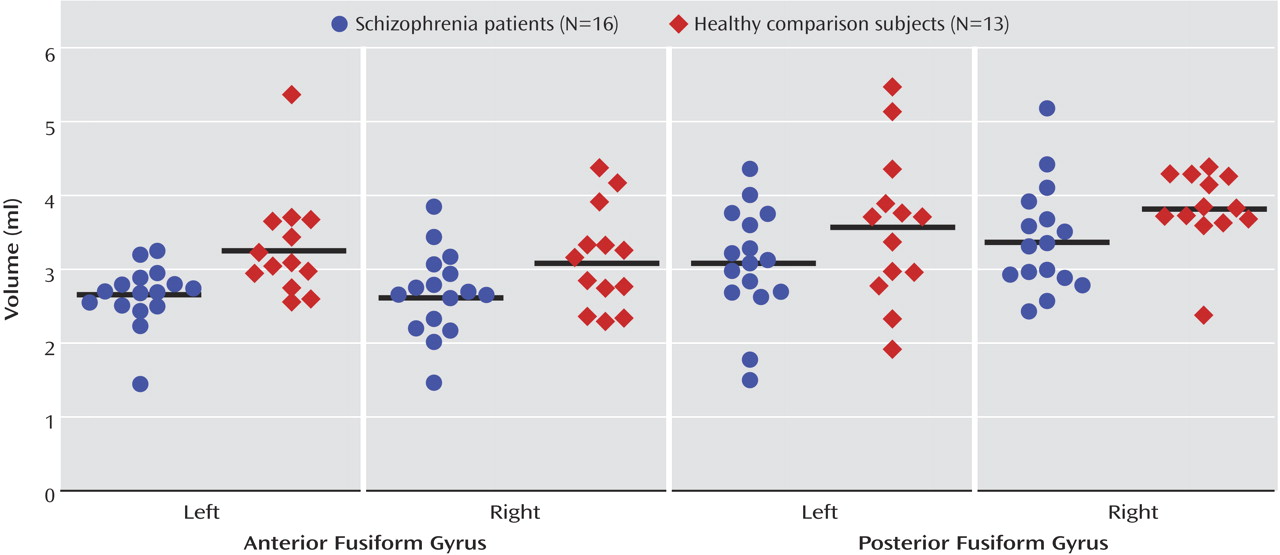
The scattergrams in
Figure 3 show the anterior and posterior fusiform gyrus absolute volumes on the left and right side in 16 patients with schizophrenia and 13 healthy comparison subjects. The three-factor ANCOVA of the standardized region-of-interest values (z scores) revealed a significant main effect of group (F=6.21, df=1, 27, p=0.02) but no significant main effects of hemisphere (F=2.61, df=1, 27, p=0.12) or subregion (F=0.10, df=1, 27, p=0.76) and no significant group-by-hemisphere-by-subregion (F=0.37, df=1, 48, p=0.55), group-by-hemisphere (F=0.01, df=1, 27, p=0.93), or group-by-subregion interactions (F=0.16, df=1, 27, p=0.70). These results suggest that patients with schizophrenia have bilaterally reduced anterior and posterior fusiform gyrus gray matter volumes, relative to healthy comparison subjects.
Correlations Between Fusiform Gyrus Volume and N170 Amplitude
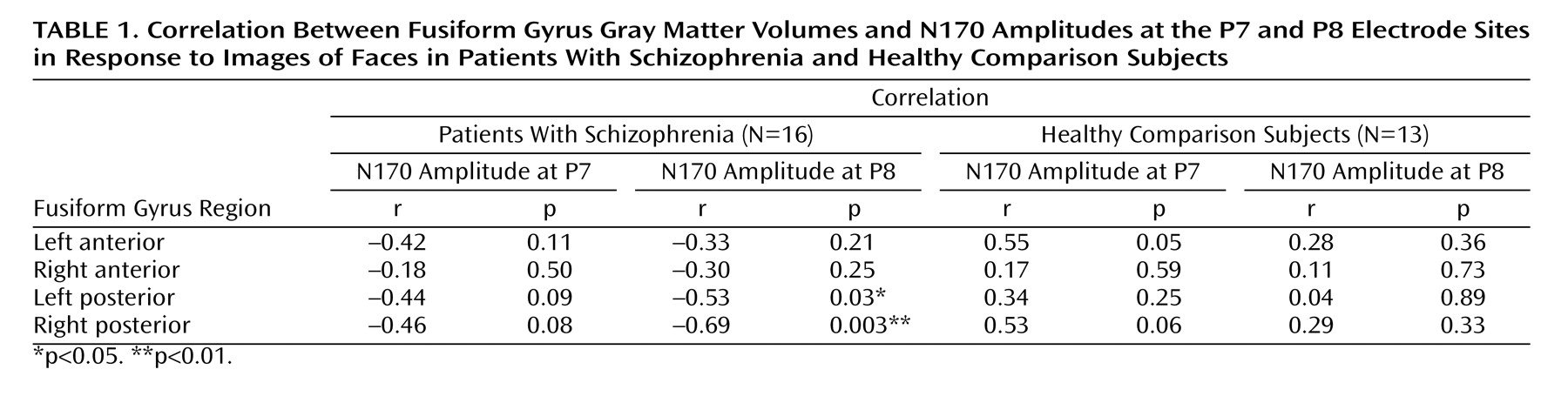
For the schizophrenia patients, significant negative correlations were found between right posterior fusiform gyrus volumes and N170 amplitudes in response to images of faces at P8 (r=–0.69, N=16, p=0.003) and PO10 (r=–0.52, N=16, p=0.04) and between left posterior fusiform gyrus volumes and N170 amplitudes in response to images of faces at P8 (r=–0.53, N=16, p=0.03). However, no significant correlations were found between fusiform gyrus volumes and N170 amplitudes in response to images of cars and hands (–0.48≤r≤–0.01, N=16, 0.05<p≤0.97). For the healthy comparison subjects, no significant correlations were found between fusiform gyrus volumes and N170 amplitudes in response to any stimuli (–0.11≤r≤–0.02, N=13, 0.73≤p≤0.95).
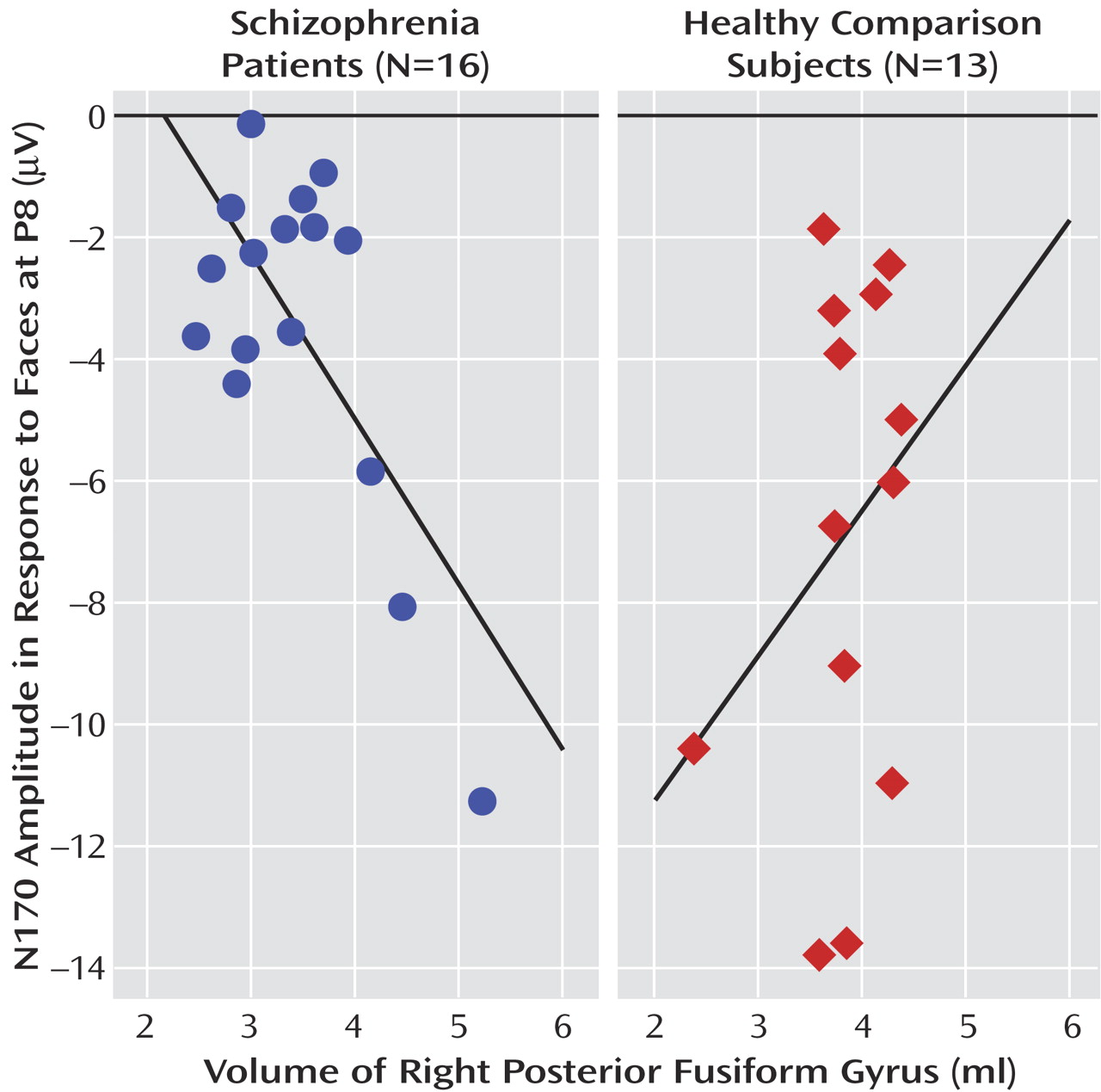
Table 1 shows the results of Pearson product-moment correlations of absolute fusiform gyrus volumes and N170 amplitudes at P7/P8 in response to images of faces for both groups. The scattergrams in
Figure 4 show the relationship between right posterior fusiform gyrus volumes and N170 amplitudes at P8 in response to images of faces in both groups. The correlation coefficients for this relationship were significantly different between groups (z=2.7, p=0.006).
To further explore the specificity of the relationship between N170 amplitudes and fusiform gyrus subregion volumes, we performed multiple linear regression analyses for the correlations that were found to be significant. In each group, the relative volumes of fusiform gyrus subregions were the dependent variables, and the N170 amplitudes in response to images of faces, cars, and hands at each electrode site with a significant correlation of N170 amplitude and fusiform gyrus volume were the independent variables. The results indicated that N170 amplitude at P8 (but not at any other site) in response to images of faces was a significant predictor of right posterior fusiform gyrus volume in the schizophrenia patients (β=–0.18, SE=0.07, t=–2.69, p=0.02) and that N170 amplitudes in response to images of hands and to images of cars were not significant predictors of right posterior fusiform gyrus volume (β=–0.04, SE=0.12, t=–0.37, p=0.72 and β=0.09, SE=0.09, t=0.90, p=0.39, respectively). In addition, N170 amplitudes at P8 in response to any stimuli were not significant predictors of left posterior fusiform gyrus volume in the schizophrenia patients (response to faces: β=–0.04, SE=0.05, t=–0.77, p=0.46; response to hands: β=0.02, SE=0.09, t=0.23, p=0.82; response to cars: β=–0.06, SE=0.07, t=–0.74, p=0.48). We interpreted these results as further evidence supporting the specificity of the relationship between N170 amplitude in response to images of faces, but not other types of stimuli, and volumetric measures of the right posterior fusiform gyrus.
Discussion
In the current study we investigated the N170 visual evoked potential elicited by objects (including faces), fusiform gyrus gray matter volume assessed with high-resolution MRI, and their interrelationship in schizophrenia patients and healthy comparison subjects. We found that 1) schizophrenia patients, relative to healthy comparison subjects, showed bilateral N170 amplitude reduction specifically in response to images of faces (it is important to note that no stimulus effect was observed in the patient group); 2) patients, relative to healthy comparison subjects, had smaller bilateral anterior and posterior fusiform gyrus gray matter volumes; and 3) for patients, but not for healthy comparison subjects, there was a significant negative correlation between right posterior fusiform gyrus volume and N170 amplitude at right posterior temporal electrode sites in response to images of faces but not to images of other objects.
This study’s finding of an association between smaller gray matter volume in fusiform gyrus and N170 amplitude reduction in schizophrenia strengthens the evidence linking the fusiform gyrus to the N170 potential, and the results are consistent with a major role of the fusiform gyrus in generation of the N170 potential. This result underlines the utility of linking in vivo MRI volumetric evaluation and measurement of event-related potentials to find an association between putative anatomical substrates and event-related potentials, as the authors have found in a previous evaluation of the superior temporal gyrus and the P300 potential
(21). In the previous study also, only the schizophrenia patients showed a significant correlation between the event-related potential and the MRI findings. We believe that deficits in gray matter volume may contribute to pathological findings for event-related potentials in schizophrenia. In the present study, we cannot exclude contributions to the N170 potential from other anatomical regions, because not all anatomical regions that might be considered as its putative generators were evaluated. Moreover, although the correlational analyses indicated an association between both left and right posterior fusiform gyrus volumes and N170 amplitude at P8, the multiple regression analyses pointed only to an association between right posterior fusiform gyrus volume and N170 amplitude. This discrepancy may be related to a strong correlation between left and right fusiform gyrus volumes, a less tight relationship between the left posterior fusiform gyrus volume and N170 amplitude, or both.
The N170 potential is considered to be an index of the analysis of face information
(8). A recent EEG study reported that schizophrenia patients showed less difference in N170 amplitudes in response to images of faces versus images of buildings than did healthy comparison subjects
(22). In the present study, the patients with schizophrenia showed significant bilateral N170 amplitude reduction in response to images of faces, compared with the healthy subjects, but not in response to images of cars. These data suggest that schizophrenia may be associated with deficits in face perception that are already evident in the early stages of visual processing and are perhaps related to the extraction of the identity of the individual face. This deficit was clearly specific to the response to faces, versus cars, although the patients also showed an N170 amplitude reduction that approached significance in response to images of another body part, hands. Furthermore, when the specificity of the association between N170 amplitude and subregion volumes in the fusiform gyrus was probed in a multiple linear regression analysis, we found that N170 amplitude in response to images of faces, but not in response to images of hands or cars, was a predictor of right posterior fusiform gyrus volume in schizophrenia patients. These findings further support the specificity of the structure-function association between fusiform gyrus volume reduction and N170 amplitude in response to faces.
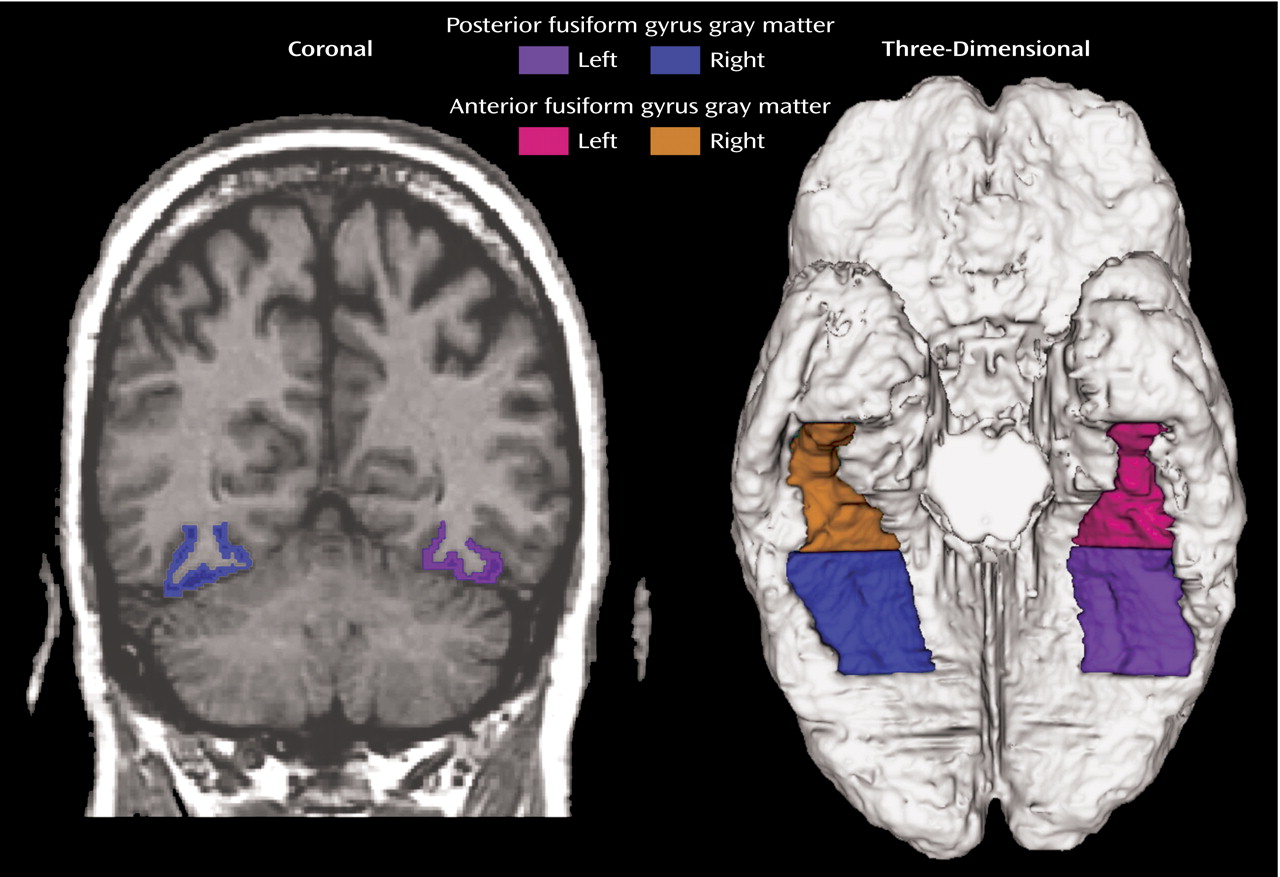
It has been suggested that a specific brain processing network that is present early in development underlies face perception
(23,
24). Evidence from fMRI studies has related face processing to activation in the region defined here as the posterior fusiform gyrus (
Figure 1), with Kanwisher et al.
(5) reporting that the middle fusiform gyrus responded selectively to faces. They termed this region the “fusiform face area” and suggested that their data supported neither a generic within-category identification role of the fusiform gyrus nor an objects-of-expertise interpretation
(25). Our data in patients with schizophrenia, who showed a pathological deficit in fusiform gyrus volume, also support the role of fusiform gyrus in face perception. Significant correlations between fusiform gyrus volumes and N170 amplitudes in the patients were observed only for responses to images of faces and not for responses to other stimuli, indicating a deficit in the anatomical substrate involved in face processing. This anatomical deficit, at the cellular level, may consist of a reduction of the dendritic tree, the strongest source of event-related potentials.
Some fMRI studies have suggested that the right fusiform face area, which is also associated with face perception, can be recruited in the processing of objects that subjects are highly familiar with, as in cases of expert knowledge
(26,
27). After repeated training, EEG findings similar to those associated with responses to faces have been reported in response to other objects
(28,
29). Because we did not measure the degree of “expert knowledge” in processing stimuli
(19,
26), we cannot be certain whether the observed N170 amplitudes and fusiform gyrus deficits reflect a disturbance of an innate system for face processing or a disturbance of the capability for developing “expert knowledge.” However, to our knowledge, our data provide the first combined functional and anatomical evidence for disturbed face processing in schizophrenia.
The limitations of our study must be considered in interpreting the conclusions. First, we were unable to rule out the contribution of chronic neuroleptic treatment to abnormalities in the N170 potential (although no significant correlations between N170 amplitudes and neuroleptic doses were observed) nor, in the present group of subjects, could we demonstrate the specificity of N170 abnormalities to schizophrenic psychosis as contrasted to affective psychosis. It will thus be important to investigate whether N170 abnormalities are observed in patients with schizophrenia and affective psychosis at first hospitalization, when most patients have a minimal or no medication history. Second, because this study did not include female subjects, we do not know if female patients show the N170 abnormalities observed in this study.
In summary, patients with schizophrenia showed significant bilateral N170 amplitude reduction in response to images of faces, compared to healthy subjects. In addition, right posterior fusiform gyrus volumes were significantly correlated with N170 amplitudes in response to faces at the right posterior temporal electrode sites. These results provide evidence for deficits in the neural substrate for face processing in patients with schizophrenia.