Reduced volume and neuronal density have been observed in the thalamus of individuals with schizophrenia, with several studies suggesting that specific thalamic nuclei are affected
(1–
5). In previous work, we observed inward deformities of the thalamic surface in areas proximal to the anterior nuclei, the medial dorsal nucleus, and the pulvinar
(6). In addition, individuals with schizophrenia exhibit decreased cognitive task-related functional activation within similar locations, although the specific nuclei that exhibit these alterations are less clear, perhaps because of the coarser spatial resolution of functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) compared to structural MRI
(7,
8). Previous [
18F]fluorodeoxyglucose PET studies have reported decreased relative glucose metabolism in individuals with schizophrenia in relation to healthy comparison subjects in larger single nuclei, such as the medial dorsal nucleus, during a serial verbal learning task
(9,
10). However, because these studies examined only a task activation state and no “control” state, it is not clear to what extent the changes in metabolism were task-related.
The results of prior studies of the thalamic complex in individuals with schizophrenia highlight the importance of dividing the thalamus into smaller, more homogeneous regions of interest. With the advent of improved scanning techniques, the study of precise relationships between structural, functional, and neurocognitive impairments in brain regions, such as the thalamus, is now feasible
(11). Close examination of the projections of individual thalamic nuclei reported to be altered in schizophrenia may help to elucidate the role of thalamic abnormalities in the working and episodic memory deficits found in such individuals. For example, the intermediate part of the medial dorsal nucleus projects to the dorsomedial and dorsolateral prefrontal cortex, cortical regions particularly important for working and episodic memory, and reductions in the magnitude of correlations between the [
18F]fluorodeoxyglucose PET signal in the medial dorsal nucleus and these prefrontal regions have been recently reported
(12). Individuals with schizophrenia exhibit structural deficits within the prefrontal cortex as well as reduced functional activation during encoding and retrieval memory tasks
(8,
13–16). Other memory-related structures, such as the hippocampus and the entorhinal cortex, together with the prefrontal cortex are reciprocally connected to the anterior nuclei
(17). Deformations in hippocampal structure are a robust characteristic of schizophrenia and have been associated with episodic memory deficits
(18–
21). Finally, the pulvinar projects to several cortical areas, including sensory association areas, limbic areas, and the prefrontal cortex, and disruption of the pulvinar function produces deficits in verbal and nonverbal memory
(17,
22).
The functional processes engaged by projection sites of the anterior nuclei, the medial dorsal nucleus, and the pulvinar suggest that abnormalities of specific thalamic nuclei may contribute to memory deficits in individuals with schizophrenia. Indeed, Andreasen and colleagues
(7,
23) attributed symptoms of “cognitive dysmetria” or the “disruption of the fluid, coordinated sequences of thought and action that are the hallmark of normal cognition,” to impairments in the cortical-cerebellar-thalamic-cortical circuit. Within this circuit, the thalamus mediates the connections between the cerebellum and cortical areas, including the prefrontal cortex and sensory and motor cortices.
The current study had two main goals. Our first goal was to test the hypothesis that cognitive task-related functional activation in schizophrenia subjects is selectively impaired in thalamic nuclei previously shown to exhibit structural abnormalities, namely the anterior nuclei, the medial dorsal nucleus, and the pulvinar. We also intended to investigate potential relationships between individual differences in thalamic functional activation, thalamic volume or shape, and performance on working memory and episodic memory tasks in individuals with and without schizophrenia.
Method
Subjects
Sixty-four individuals participated in the study: 1) 36 individuals diagnosed with schizophrenia according to DSM-IV and 2) 28 healthy comparison subjects. The participants were a subset of individuals who participated in prior studies investigating the structure of the thalamus
(6) and task-related changes in functional brain activation
(8). Detailed recruitment and diagnostic information can be found in previous publications
(6,
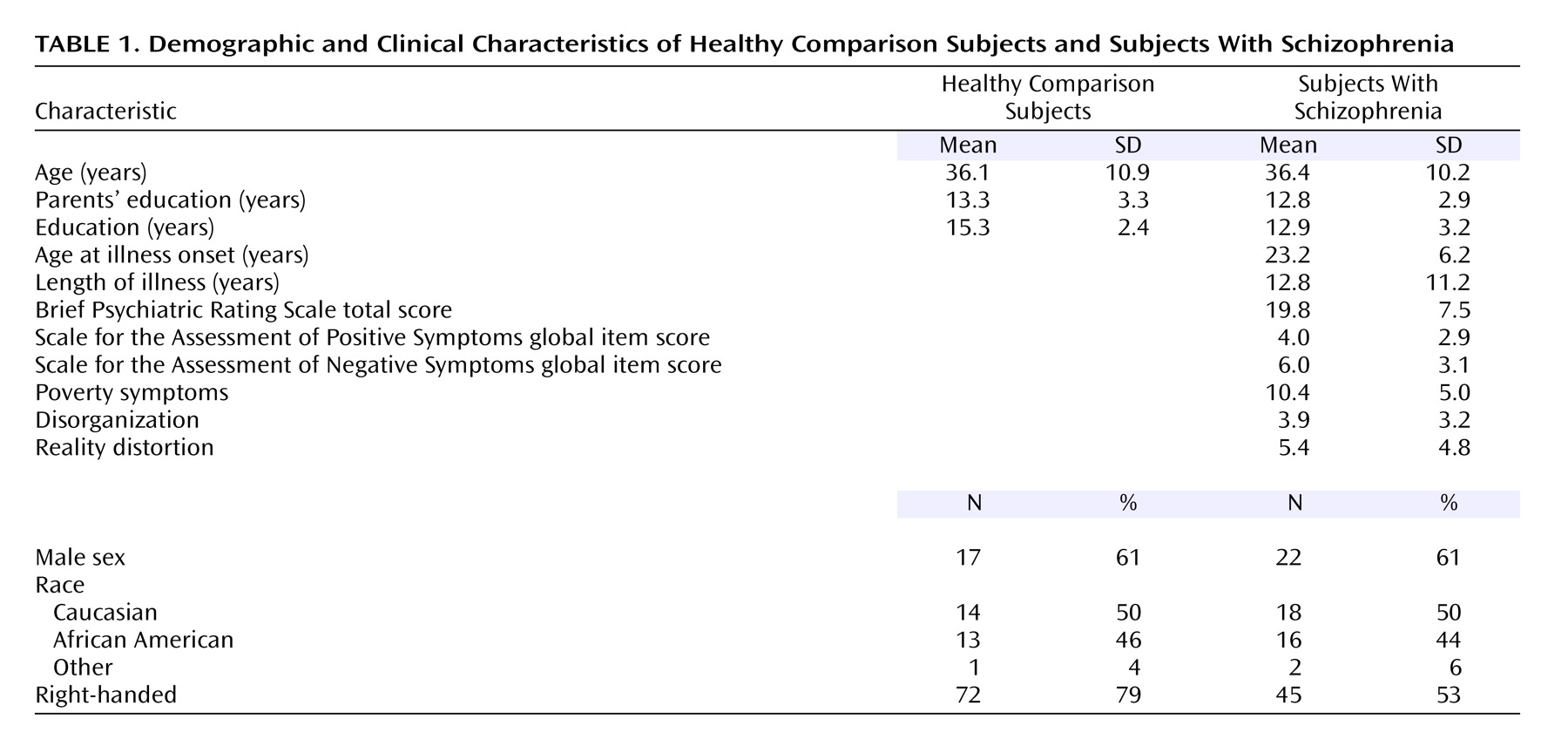
8). All of the participants with schizophrenia were medicated, with 21% receiving typical and 79% receiving atypical antipsychotics. All of the subjects underwent clinical and diagnostic assessments administered by a trained research assistant, gave written informed consent to participate after receiving a complete description of the study, and were monetarily compensated for their time. Demographic characteristics of each group are shown in
Table 1. The schizophrenia and comparison subjects did not differ significantly by age, gender, race, handedness, or parental socioeconomic status. However, the comparison group completed, on average, significantly more years of education (mean=15.3 years, SD=2.4) than the schizophrenia group (mean=12.9 years, SD=3.2) (unpaired t=3.3, df=62, p<0.002).
Thalamic Structural Measures
Structural magnetic resonance (MR) brain images were acquired for all subjects by using a turbo-fast low-angle shot sequence (TR=20, TE=5.4, flip angle=30°, number of acquisitions=1, matrix=256×256, voxel size=1 mm
3, scanning time=13.5 minutes)
(24), as previously reported
(6). A separate template scan was used to map the surface of the template thalamus to all individual subjects by large-deformation high-dimensional brain mapping. Whole thalamic volumes and shape eigenvectors that described principal dimensions of thalamic shape variation within the subject groups were computed from the mapped individual thalamic surfaces. A linear combination of three of these eigenvectors was previously used to discriminate schizophrenia and comparison subjects
(6), and estimates of the linear predictor (xbeta) were computed for each subject (SAS, v8, SAS Institute, Cary, N.C.) as thalamic shape scores.
Thalamic Functional Measures
A 1.5-T Siemens VISION system MRI scanner (Erlangen, Germany) and an asymmetric spin-echo, echo-planar sequence with T
2* blood-oxygenation-level-dependent (BOLD) contrast (TR=2.5 sec, TE=50 msec, field of view=24 cm, flip=90°) were used to collect functional images during each task. We acquired 102 sets of 16 contiguous axial images, 8-mm thick, oriented parallel to the anterior commissure-posterior commissure plane (3.75×3.75 in-plane resolution) during each functional run. Functional data were processed as described in Barch et al.
(8). The size of each voxel comprising the functional scans (3 mm
3/voxel) was appreciably larger than that of the structural scans (1 mm
3/voxel).
Task and Materials
As previously reported
(8), each subject completed three types of tasks during the functional scanning session. One task was a working memory task (a two-back version of the N-back task) in which the participants pressed a target button each time a visual stimulus was identical to the stimulus presented two trials previously and a nontarget button otherwise. Another task was an intentional encoding task in which the subjects were instructed to remember each of a series of visual stimuli for later probing in the third type of task and simultaneously performed a simple motor task to control for motor responses present in the other two tasks. In a third yes/no recognition task, the subjects pressed a target button if they encountered a particular visual stimulus during the encoding task and a nontarget button if the stimulus was novel. The participants performed each of these tasks twice: once with words (verbal) and once with faces used as stimuli. Each task run consisted of four task blocks (40 seconds each) interleaved with three fixation blocks (25 seconds each). Each task block was composed of 16 trials (with stimuli presented for 2 seconds followed by a 500-msec interstimulus interval), whereas individual fixation blocks consisted of 10 trials. Four additional fixation trials were presented at the beginning and end of each run for a total of 102 trials.
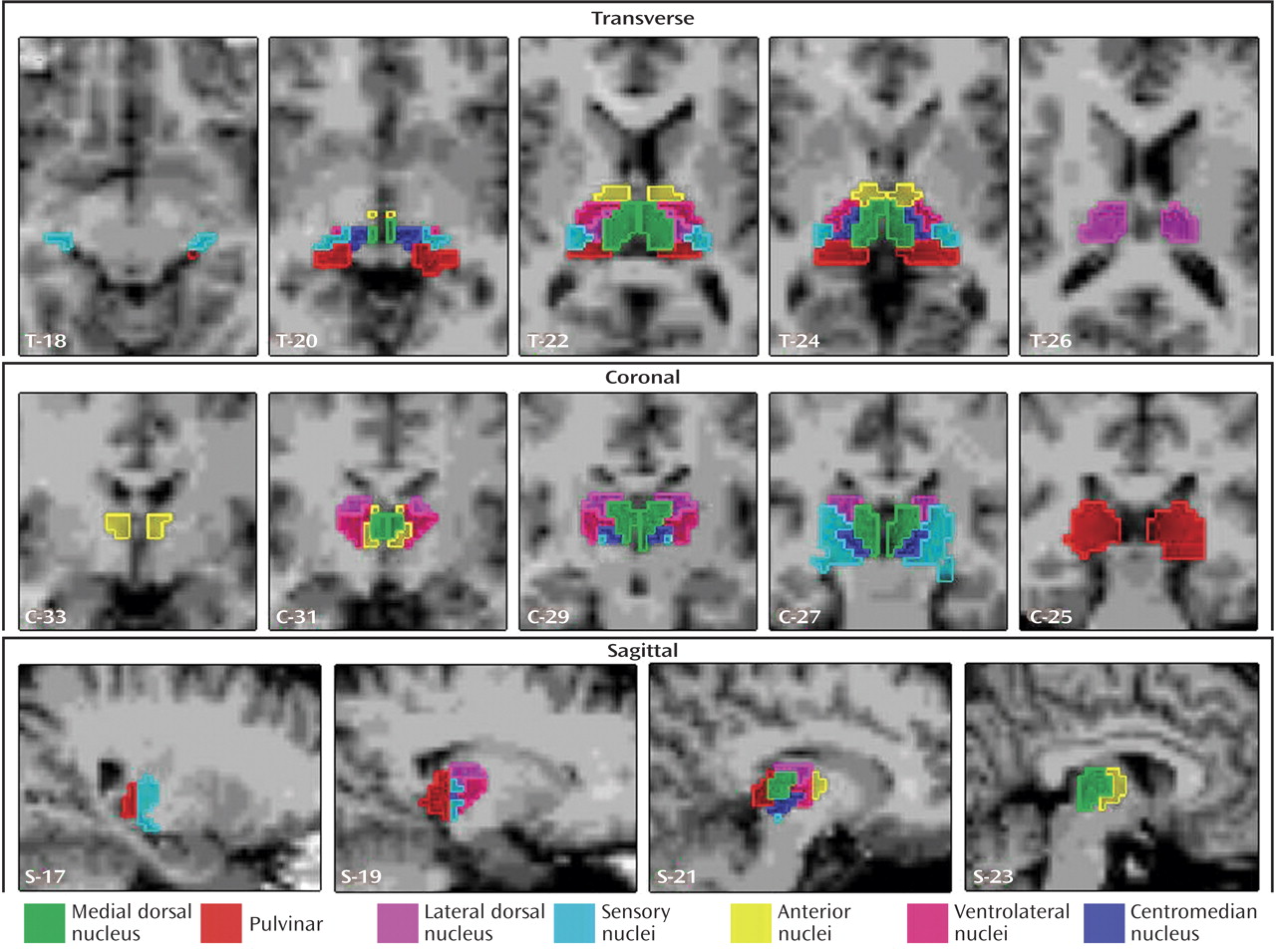
Thalamic Nuclei Region of Interest
To accommodate the larger voxel size of functional MR voxels compared to structural MR voxels, thalamic regions of interest were manually outlined in 1×1×1 mm
3/vox resolution in the structural template scan by using Talairach coordinates and Analyze-AVW software (Mayo Medical Foundation, Rochester, Minn.) by a team of experts (L.W. and M.H.G.) and then warped into 3×3×3 mm
3/vox resolution. The delineation of neuroanatomical boundaries was derived from myelin-stained coronal reference sections
(25) and relevant anatomical and functional criteria
(17). We generated the following seven thalamic regions of interest corresponding to individual nuclei or groupings of individual nuclei: the anterior nuclei (anterior combined with ventral anterior), the centromedian nucleus, the medial dorsal nucleus, the lateral dorsal nucleus, the sensory nuclei (medial and lateral geniculate, ventral posterior lateral, and ventral posterior medial combined), the pulvinar, and the ventrolateral nuclei (
Figure 1). We grouped the two small anterior and ventral anterior nuclei together as the “anterior nuclei” because of their anatomical proximity and functional similarity. We also grouped the medial geniculate, lateral geniculate, ventral posterior lateral, and ventral posterior medial nuclei into one region of interest called the “sensory nuclei” based on anatomical proximity and functional similarities. The medial dorsal nucleus and the pulvinar were treated as individual regions of interest because they exhibit dissimilar projections to a variety of cortical regions, although each also projects to the prefrontal cortex. Finally, the centromedian nucleus and the ventrolateral nucleus were treated as separate regions of interest because they are functionally and anatomically unique.
Statistical Analysis
After the regions of interest were warped into 3×3×3 mm space and mapped onto each subject’s structural brain image, we used a general linear model to estimate the magnitude of task-related activity in each voxel within a region of interest for both the task and fixation blocks for each type of task separately and for each material type separately. The dependent variable for each region of interest was the average of the differences between task and fixation magnitudes across all voxels with a region of interest for each task. Analyses of variance (ANOVAs), t tests, and linear regressions with a p value threshold of 0.05 for each effect were used appropriately, and the participants were treated as a random factor.
Results
Functional Differences in Individual Thalamic Nuclei
Whole-brain exploratory analyses of group differences in task-related activation with a superset of individuals in the current study were published elsewhere
(8). The results of this previous study demonstrated functional deficits in the schizophrenia group near the dorsolateral prefrontal cortex, the thalamus, the parietal cortex, and the medial temporal lobe as well as thalamic deficits that survived corrections for multiple comparisons typical of whole-brain voxelwise analyses. The current study extended this finding by dividing the thalamus into individual regions of interest.
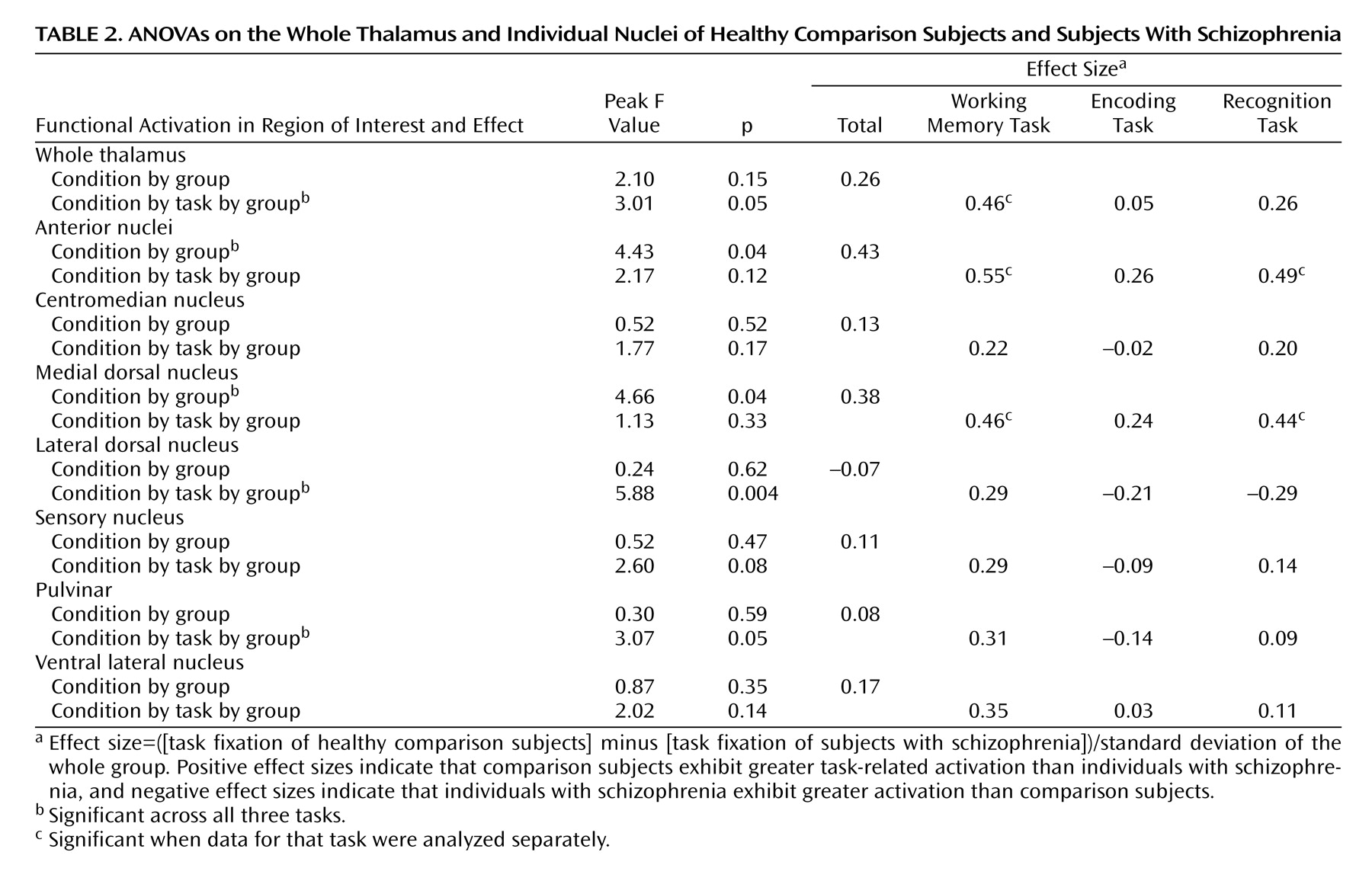
We examined group differences in the magnitude of activation in the thalamus as a whole and within each of the seven regions of interest by using ANOVAs with group as the between-subject factor and hemisphere (left, right), task (working memory, encoding, recognition), and material type (face, word) as within-subject factors. Across all three tasks, the comparison subjects showed significantly greater activation than the individuals with schizophrenia bilaterally within the anterior nuclei and the medial dorsal nucleus regions of interest (
Table 2). Post hoc analyses indicated that the group differences in task-related activation in these regions of interest were significant for both working memory and recognition tasks but not for encoding. Of importance, when we examined subgroups of schizophrenia and comparison subjects matched for working memory performance (25 comparison subjects and 32 schizophrenia subjects), we found similar effect sizes for the group differences in working memory activation in both the anterior nuclei (effect size of 0.50 versus 0.55 for the whole group) and the medial dorsal nucleus (effect size of 0.43 versus 0.46 for the whole group). Furthermore, when we examined subgroups of schizophrenia participants and healthy comparison participants matched for recognition performance (23 comparison subjects and 24 schizophrenia subjects), we again found similar effect sizes for the group differences in recognition task activation in both the anterior nuclei (effect size of 0.46 versus 0.49 for the whole group) and the medial dorsal nucleus (effect size of 0.37 versus 0.44 for the whole group).
In addition, we observed condition-by-group effects within the bilateral whole thalamus and task-by-group-by-condition effects in the bilateral whole thalamus, the lateral dorsal nucleus, and the pulvinar. In the lateral dorsal nucleus and the pulvinar, the individuals with schizophrenia exhibited less task-related activation than the comparison subjects during the working memory tasks but greater activation than the comparison group during the encoding and recognition tasks. However, as shown in
Table 2, when the regions were analyzed separately by task, none of the group differences were significant. Finally, within the whole thalamus, the comparison subjects showed significantly greater task-related activation than the participants with schizophrenia for the working memory task but not the encoding or the recognition tasks (
Table 2). When we again examined subgroups of individuals with schizophrenia and healthy comparison subjects matched for working memory performance, we found similar effect sizes for the group differences in working memory activation in the whole thalamus (effect size of 0.35 versus 0.46 for the whole group). We did not observe any hemisphere-by-group effects.
We next examined whether there were relationships between individual differences in thalamic volumes and thalamic shape (i.e., xbeta scores) and individual differences in the magnitude of task-related functional activation. We did not find any significant correlations between total thalamic volume or xbeta scores of thalamic shape and any of the thalamic functional activation measures associated with working memory, encoding, or retrieval processes.
Measures and Task Performance
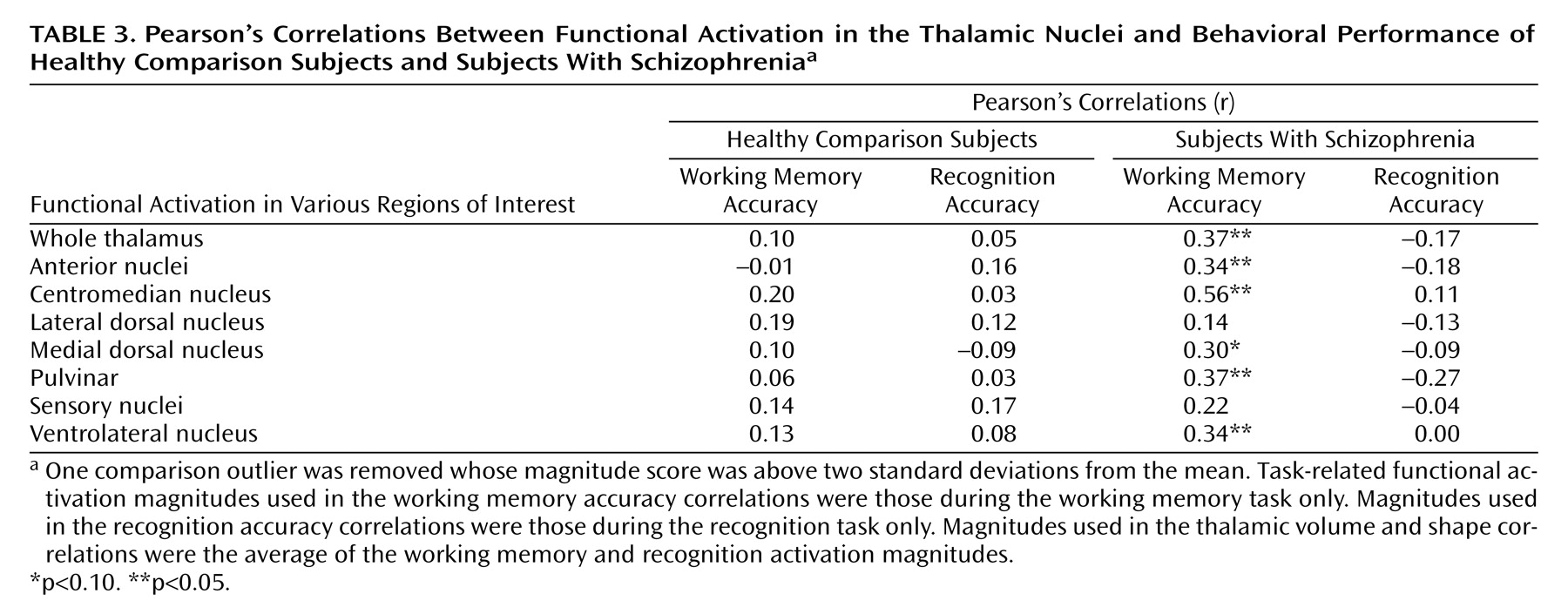
Correlation coefficients between functional magnitudes within each region of interest and the subjects’ behavioral performance on the working memory and recognition tasks are shown in
Table 3. Task-related activation was not correlated with behavioral performance in the healthy comparison group; however, there were significant or nearly significant correlations for the whole thalamus, the anterior nuclei, the centromedian nucleus, the medial dorsal nucleus, the pulvinar, and the ventrolateral nucleus within the schizophrenia group for the working memory tasks but not for the recognition tasks. All correlations are positive, suggesting that increased activation corresponded to better cognitive task performance. There were no significant correlations between thalamic shape or volume indices and task performance in either group (r ranged from –0.21 to 0.22).
Discussion
The results of the current study demonstrate that individuals with schizophrenia exhibit decreased task-related functional activation in two thalamic regions of interest (the anterior nuclei and the medial dorsal nucleus) previously implicated in the structural deformation of the thalamic complex
(2,
3,
6,
26). In contrast, we did not find similar changes in pulvinar activation. Furthermore, we demonstrated that individual differences in behavioral performance were associated with individual differences in cognitive task-related thalamic activation but not measures of thalamic shape or volume in participants with schizophrenia. Our findings of performance-independent decreased task-related functional brain activation in the anterior nuclei and the medial dorsal nucleus are consistent with the reports from a number of previous structural studies. Several other researchers have observed smaller volumes of the anterior nuclei and/or the medial dorsal nucleus in individuals with schizophrenia in relation to comparison subjects
(2,
26,
27), and in a previous study of a superset of our subjects, we noted shape deformities of the surface of the thalamic complex proximal to these nuclei
(6). Furthermore, postmortem studies of individuals with schizophrenia have reported reduced neuronal numbers within the thalamus and especially within the medial dorsal nucleus
(3–
5,
28,
29).
Contrary to our expectations, we did not see clear evidence for reduced task-related activation in the pulvinar across all tasks. However, this region exhibited a significant task-by-condition-by-group effect (
Table 2). There was a positive effect size in the working memory task, with healthy comparison participants showing greater activation than participants with schizophrenia, but this effect was not significant when the working memory task was analyzed separately. The selection of tasks and spatial resolution limitations of fMRI may have contributed to our lack of expected findings within the pulvinar. The region of interest referred to as the pulvinar is actually a collection of subnuclei (medial, lateral, inferior, and anterior pulvinar), each of which exhibits unique connections
(17). If abnormalities among individuals with schizophrenia are restricted to only some of these subnuclei, our examination of the pulvinar as a single region of interest may have masked group differences in the task-related BOLD response within specific subregions. Because the use of different cognitive tasks may recruit a different set of thalamic nuclei, our results should be considered specific to tasks assessing working and episodic memory functions.
Our finding that greater working memory-related activation in the whole thalamus, the anterior nuclei, the medial dorsal nucleus, and the pulvinar was associated with better task performance among individuals with schizophrenia is consistent with the hypothesis that circuits between these nuclei and the prefrontal cortex are critical for intact working memory performance
(8,
13–15). We also observed significant relationships within the ventrolateral nuclei and the centromedian nucleus. Although we did not correct for multiple comparisons, and unpredicted findings should be cautiously interpreted, these correlations were quite robust (
Table 2). We should further note that a subgroup of individuals with schizophrenia matched to comparison subjects for performance still demonstrated reduced anterior nuclei and medial dorsal nucleus activation. Thus, although behavioral performance and activation in these regions are clearly linked, the reduced activation in individuals with schizophrenia did not appear to be secondary to poorer behavioral performance.
We did not see similar correlations between activation in any of the thalamic nuclei and recognition task performance. This was somewhat surprising given the literature suggesting that both prefrontal cortex and hippocampus regions are important for episodic as well as working memory
(30–
33) and because we found evidence for decreased task-related activation in both the medial dorsal nucleus and the anterior nuclei for the recognition task as well as the working memory task. Although these findings suggest that the cortical-cerebellar-thalamic-cortical circuit may be more critical for working memory performance than for episodic memory performance, prospective examination in future studies is needed.
We did not observe correlations between individual differences in total thalamic volume or thalamic shape and task-related functional activation. However, the functional activation disturbances and shape abnormalities were not equally present throughout the whole thalamus but, rather, more apparent in some nuclei than others. Thus, whole-thalamus volume and shape measures may not reveal structural-functional correlations that might exist for individual nuclei. Using high-resolution MRI, future research should be directed at quantifying the shape differences specifically within these individual regions so that correlations between the degree of shape differences and behavior or function can be investigated in more detail.
In summary, individuals with schizophrenia showed reductions in thalamic activation during memory tasks within the whole thalamus, the anterior nuclei, and the medial dorsal nucleus. These results remained significant after matching the groups with respect to task performance. We also observed correlations between functional activation within the anterior nuclei, the medial dorsal nucleus, and other nuclei and behavioral performance on memory tasks in the individuals with schizophrenia. However, we did not find a significant relationship between global measures of thalamic structure and functional activation or behavioral performance. Future research should be aimed at quantifying structural measurements within individual thalamic nuclei so that we can investigate relationships between structure, function, and cognition within particular subregions of the thalamic complex and other regions affected in schizophrenia. Furthermore, because nonpsychotic relatives of schizophrenic subjects also seem to exhibit reduced thalamic volumes and neurocognitive deficits that are accompanied, interestingly, by greater task-related functional activation in the prefrontal cortex, the anterior nuclei, and the medial dorsal nucleus, it would be interesting to characterize such relationships in nonpsychotic relatives of individuals with schizophrenia
(34,
35).