Erectile dysfunction secondary to antipsychotic medication is a significant clinical problem faced by people with schizophrenia taking such treatments
(1,
2). In addition to a reduction in the quality of life, it often results in medication noncompliance
(3,
4). The need for long-term prophylaxis with antipsychotic medication in such individuals suggests the need for specific treatments to overcome the dysfunction.
Phosphodiesterase-5 inhibitors are currently the first-line treatment for erectile dysfunction
(5–
7). Although phosphodiesterase-5 inhibitors would be expected to be effective for erectile dysfunction in this population, and there are positive anecdotal reports regarding this medication, no placebo-controlled data have been published
(8–
10), to our knowledge. In addition, its use in erectile dysfunction caused by hyperprolactinemia and/or monoaminergic dysfunction secondary to antipsychotic medication has not been systematically explored. In this study, we investigated the efficacy and tolerability of sildenafil citrate in men with antipsychotic-induced erectile dysfunction with a randomized, double-blind, placebo-controlled, flexible-dose, two-way crossover trial. The null hypothesis was that sildenafil was not superior to placebo in the treatment of antipsychotic-induced erectile dysfunction in terms of efficacy and tolerability.
Method
Setting
The Department of Psychiatry at Christian Medical College in Vellore, India, is a 100-bed tertiary referral center managing adults and children with mental and behavioral disorders. The center treats patients with a variety of disorders, including schizophrenia, affective illness, substance dependence, anxiety, and adjustment disorders. The center employs a multidisciplinary approach to the care of patients with mental illness. Two hundred to 250 outpatients are seen daily.
Subjects
Records of more than 30,000 outpatients attending the hospital during the study period (April 2002 to March 2004) were retrieved. All married male patients with schizophrenia or delusional disorder who were currently living with spouses and receiving regular treatment were interviewed (N=150) by the primary investigator (R.G.) for erectile dysfunction. Temporal correlation of erectile dysfunction with initiation of antipsychotic drugs and/or raised serum prolactin levels was used as criterion to ascertain the cause of erectile dysfunction as drug-induced. A detailed history was elicited and a physical examination was performed to exclude common medical causes of erectile dysfunction (e.g., hypertension, coronary artery disease, peripheral vascular disease, atherosclerosis, diabetes mellitus, thyroid disease, CNS tumors, spinal and abdominal surgery, and local injury). In addition, laboratory investigations (e.g., 2-hour postprandial blood glucose levels) were performed at baseline.
The following inclusion criteria were employed to recruit subjects: 1) erectile dysfunction according to DSM-IV criteria, 2) ages 20 to 45, 3) married and living with spouse, 4) diagnosis of schizophrenia or delusional disorder (ICD-10), 5) currently receiving a stable dose of an antipsychotic and other medication, and 6) erectile dysfunction judged to be secondary to antipsychotic medication as assessed by either raised prolactin level or onset of erectile dysfunction after the initiation of antipsychotics.
Exclusion criteria were 1) the regular use of nitrates, anticoagulants, or aspirin 2 weeks before recruitment; 2) comorbid depressive syndrome; 3) treatment with antidepressants; 4) alcohol or other substance dependence; 5) history of use of sildenafil citrate; and 6) continued use of other measures to improve erectile function.
All patients who met the eligibility criteria were invited to take part in the study. The details of the study were explained, and informed consent was obtained from the patient and his spouse. The trial was approved by the institutional review board.
Instruments
The Positive and Negative Syndrome Scale (PANSS)
(11) was used to document psychopathology. The International Index of Erectile Function
(12,
13) provides accurate and reliable information as a quantitative index of erectile dysfunction severity and was used to assess erectile dysfunction at baseline. The International Index of Erectile Function is highly predictive of patients’ ability to achieve satisfactory intercourse, as indicated by strong correlations with other measures of intercourse ability, such as an event log. However, the instrument requires an observation period of 1 month and, hence, could not be used because weekly assessments were planned.
The patients were instructed to keep a detailed daily diary of sexual activity. They were instructed to complete a template with a detailed and objective structure. Data from the patients’ logs were used as a primary source of assessment because sexual function is best assessed in a natural setting with self-report techniques
(14). The records included information on time, doses of study drug taken, time of onset of erection, duration and quality of erections, whether the erections were associated with sexual stimulation, occurrence of satisfactory sexual intercourse, and whether other sexual activity occurred. The patients were asked to grade the quality of erections on a 4-point scale in which 1=increase in size but not hard, 2=hard but not hard enough for penetration, 3=hard enough for penetration (but not completely hard), and 4=completely hard. The Global Efficacy Questionnaire
(5) was also employed to assess erectile function. Sociodemographic data were recorded with a specially designed proforma.
Assessments
The PANSS and the International Index of Erectile Function were employed at baseline. Sociodemographic details were also collected. The patients were then entered into a 1-week run-in period. The patients’ logs of daily sexual activity were collected every week (on days 7, 14, 21, 28, and 35). The Global Efficacy Questionnaire was used on days 21 and 35. The research psychiatrist (R.G.) carried out the screening, the PANSS, the International Index of Erectile Function, and the Global Efficacy Questionnaire assessments. The patients’ logs were assessed by two assessors (R.G. and A.K.).
Assignment and Masking
The patients recruited for the study were randomly assigned into two groups: one, which received sildenafil, followed by placebo, whereas the order of medication was reversed in the other group. The random assignment schedule was derived with a random-number table. Concealed instructions, which the research psychiatrist opened at the time of random assignment, were employed to allocate patients to either group. The research psychiatrists who executed the assignment of patients did not generate the assignment schedule. The research psychiatrists assessing outcome were blind to intervention status. The allocation code was broken after data entry was completed.
Intervention
The sildenafil citrate and placebo capsules were identical. Each sildenafil capsule contained 25 mg of the drug. The patients were instructed to take a maximum of one capsule per day for the first week. If the patient did not achieve a satisfactory erection with this dose, he was asked to increase the dose to two capsules per day for the next week. At the end of the first 2-week period, the patient then crossed over to the other treatment arm for the next 2 weeks. A similar dose strategy was employed in the placebo group. There was no washout period in between the two treatment periods because the half-life of sildenafil is 4–6 hours. No other psychosexual treatment was given during the study period.
Outcome Assessment
Data from the patients’ logs were used as a primary source of assessment of sexual function during the trial. The Global Efficacy Questionnaire was also employed to assess outcome.
Group Size Calculation
From the available literature
(7), assuming the independence of response in each patient with an anticipated proportion of discordant pairs being 0.34 and an anticipated odds ratio of 7.5 at a 5% level of significance and 80% power, the group size required was 26.
Analysis
Descriptive statistics and frequency distributions were employed to describe continuous and categorical variables, respectively. Analysis was performed on efficacy variables and included all patients who completed the trial. All tests of significance were two-sided and evaluated at a significance level of 5%. The paired t test was used to assess the significance of the following variables: 1) the number of grade 3 or 4 erections, 2) the duration of erections, 3) the number of satisfactory erections, 4) the number of doses of drug per week, 5) the number of occurrences of satisfactory sexual intercourse as a proportion of the number of doses taken, and 6) the number of satisfactory grade 3 or 4 erections as a proportion of doses taken. The odds of a satisfactory event were calculated as a/b, with a=X/(100–X), in which X was the percentage of grade 3 or 4 erections with sildenafil treatment, and with b=Y/(100–Y), in which Y was the percentage of grade 3 or 4 erections with placebo treatment.
The results of the Global Efficacy Questionnaire were evaluated with McNemar’s chi-square test. Subgroup analysis was performed for subjects with elevated serum prolactin levels. The Wilcoxon matched-pairs signed rank test was used to assess the significance in the subgroups for the following variables: 1) the number of grade 3 or 4 erections, 2) the duration of erections, and 3) the satisfaction with erections.
The analysis also examined period effect and treatment-by-period interactions
(15,
16). Effect sizes were also calculated. The kappa statistic was computed to assess the degree of blinding of the research psychiatrist. Data were analyzed with SPSS PC version 11.0.1 (SPSS, Chicago).
Poisson regression analysis was also used to assess the effects of sildenafil. The number of erections in a given week was the dependent variable, and an indicator variable (0/1) was used to represent the receipt of drug or placebo. The effects of period and week were examined with indicator variables for each. The model was also used to examine if the effect of sildenafil was different in the first period versus the second period and between the first week and second week. Generalized estimating equations (PROC GENMOD in the SAS system [SAS Institute, Cary, N.C.]) were used to obtain the variance.
Results
Recruitment of Subjects
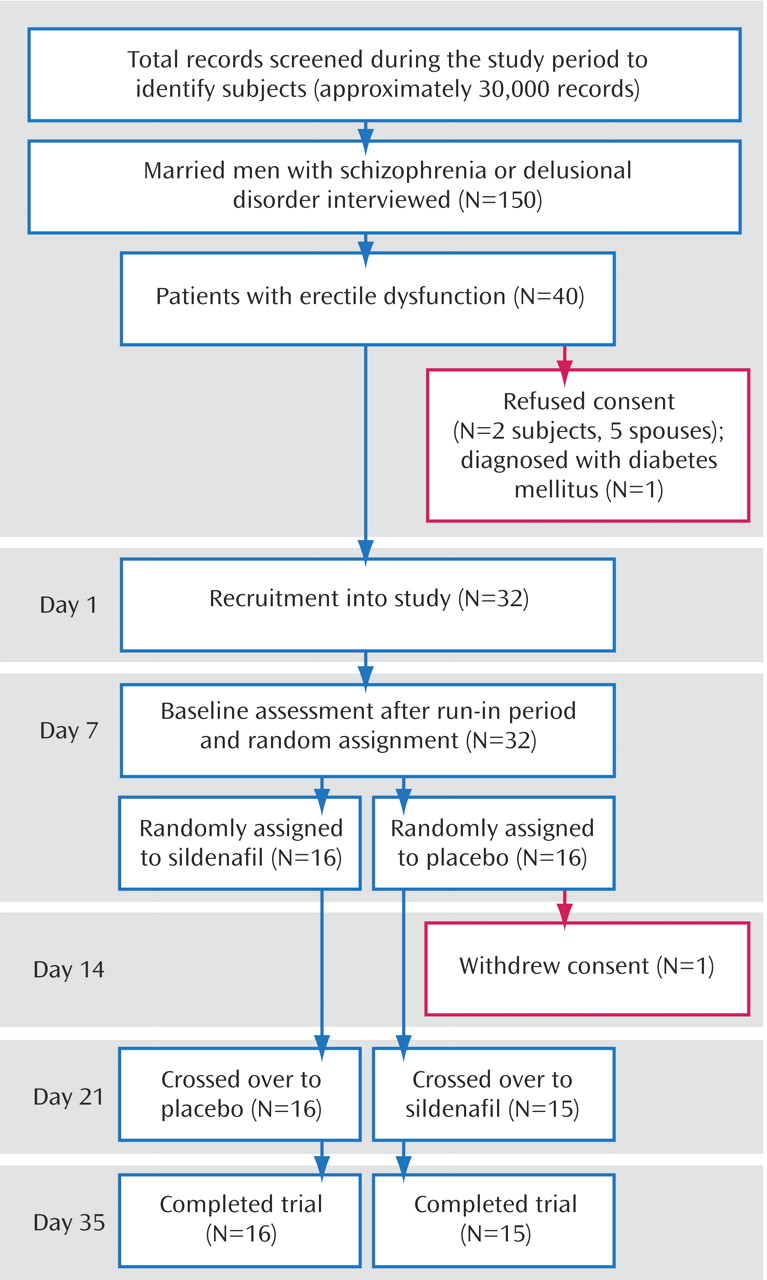
The recruitment details and follow-up details are given in
Figure 1. One subject withdrew consent immediately after randomization.
Sociodemographic and Clinical Profile
The average age of the subjects was 35.1 years (SD=5.5, range=24–45). They were married for an average duration of 10.1 years (SD=6.8, range=1–25). The mean PANSS score was 40.8 (SD=6.0, range=32–55). They were taking an average dose of 556.3 mg of chlorpromazine equivalents (SD=198.6, range=200–1000). The ICD-10 diagnostic categories were as follows: paranoid schizophrenia (N=23), catatonic schizophrenia (N=1), undifferentiated schizophrenia (N=5), and delusional disorder (N=3). They were taking the following antipsychotic medications: risperidone (N=17), olanzapine (N=12), clozapine (N=1), and fluphenazine decanoate (N=2). The mean dose of trihexyphenidyl was 1.2 mg/day (SD=1.4). Three patients were also taking diazepam.
Period Effect and Treatment-Period Interaction
The period effect was found to be insignificant for the total number of grade 3 or 4 erections (t=0.93, df=29, p>0.05), the number of times of satisfactory intercourse (t=0.86, df=29, p>0.05), and the mean duration of erections (t=–0.04, df=29, p>0.05). The treatment-period interaction was also assessed and was insignificant for the number of grade 3 or 4 erections (t=–1.23, df=29, p>0.05), the number of satisfactory times of sexual intercourse (t=–1.59, df=29, p>0.05), and the mean duration of erections (t=–0.25, df=29, p>0.05).
Follow-Up and Analysis
One patient withdrew consent immediately after random assignment. He did not take the trial medication at all. The difference on baseline characteristics between those who participated in the trial and the patient who discontinued was not statistically significant. An analysis of all patients who completed the trial was performed.
Reliability of Assessments
Two raters independently assessed the patients’ logs for grade 3 and grade 4 erections. The kappa value for agreement between the raters was 1.0. The raters also attempted to guess the treatment given based on the patients’ logs. The agreement between the actual allocation and that of the raters was moderate (rater 1: kappa=0.49, rater 2: kappa=0.49).
Efficacy of Treatment
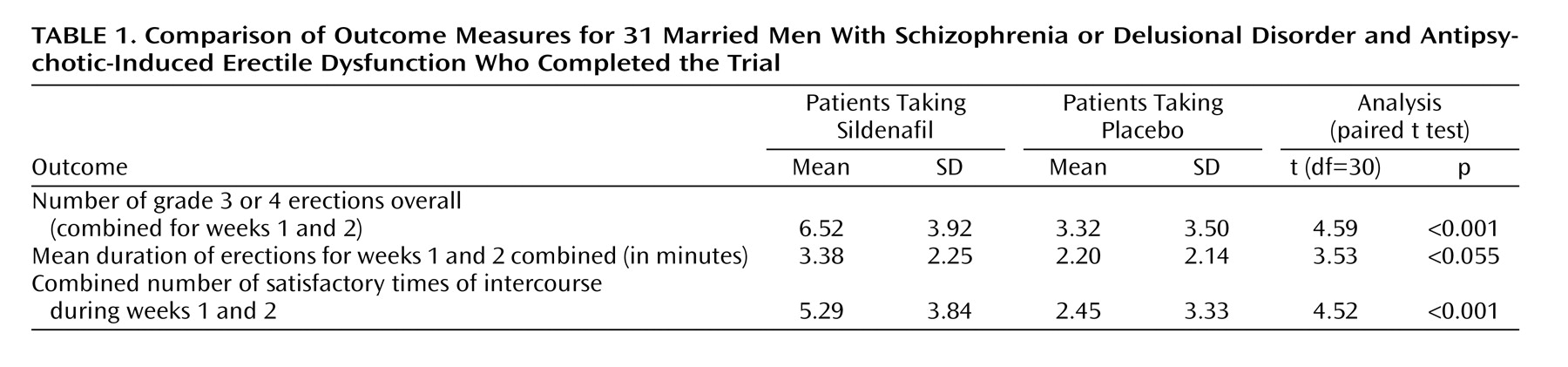
The primary and secondary outcome measures are shown in
Table 1. Sildenafil was significantly superior to placebo on all measures. The subjects also reported an increase in the number of grade 3 or 4 erections when taking sildenafil at 25 mg (t=3.04 df=30, p<0.05) and at 50 mg (t=4.04, df=30, p<0.001). They also reported an increase in the duration of erections at 25 mg (t=2.20, df=30, p<0.05) and at 50 mg (t=2.54, df=30, p<0.05) of sildenafil. Satisfaction with intercourse was also significantly better with sildenafil than with placebo at 25 mg (t=3.04, df=30, p<0.05) and 50 mg (t=4.17, df=30, p<0.001). The odds ratio of obtaining an adequate erection for sexual intercourse was 4.07 times better with sildenafil than with placebo. The odds ratio of satisfactory sexual intercourse with sildenafil was 3.77 times higher than with placebo.
Effect sizes were calculated and showed an effect size of 0.97 (95% confidence interval [CI]=0.44–1.49) for grade 3 and grade 4 erections, which would translate as six erections per week if the person took sildenafil daily. Similarly, the effect size for satisfaction with intercourse was 0.88 (95% CI=0.36–1.40) better with sildenafil than with placebo.
Modeling with Poisson regression analysis was performed to assess the effect of sildenafil on the number of grade 3 and 4 erections and for satisfactory sexual intercourse. The period and week effects and the treatment-period interactions were not significant. Sildenafil was effective in producing grade 3 and 4 erections (risk ratio=1.85, 95% CI=1.35–2.54, p<0.001). The effect was significant after adjustment for period, week, and treatment-period interactions (risk ratio=1.54, 95% CI=1.14–2.08, p<0.01) with Poisson regression. Similarly, the drug was effective in producing satisfactory sexual intercourse (risk ratio=2.16, 95% CI=1.45–3.21, p<0.001), and this remained statistically significant after adjustment for period and week effects and treatment-period interactions (risk ratio=1.80, 95% CI=1.18–2.75, p<0.01).
The Global Efficacy Questionnaire results revealed that 24 patients (77.4%) felt that sildenafil had improved their erections compared to seven patients (22.6%) who took placebo (McNemar’s χ2=12.8, df=1, p<0.001). Twenty-three patients (74.2%) felt that they would take sildenafil in the future if it were available compared to eight patients (25.8%) who took placebo (McNemar’s χ2=11.8, df=1, p<0.001). An intention-to-treat analysis with the last observation carried forward (for the patient who dropped out of the trial) showed results similar to those who completed the trial.
Safety and Tolerability
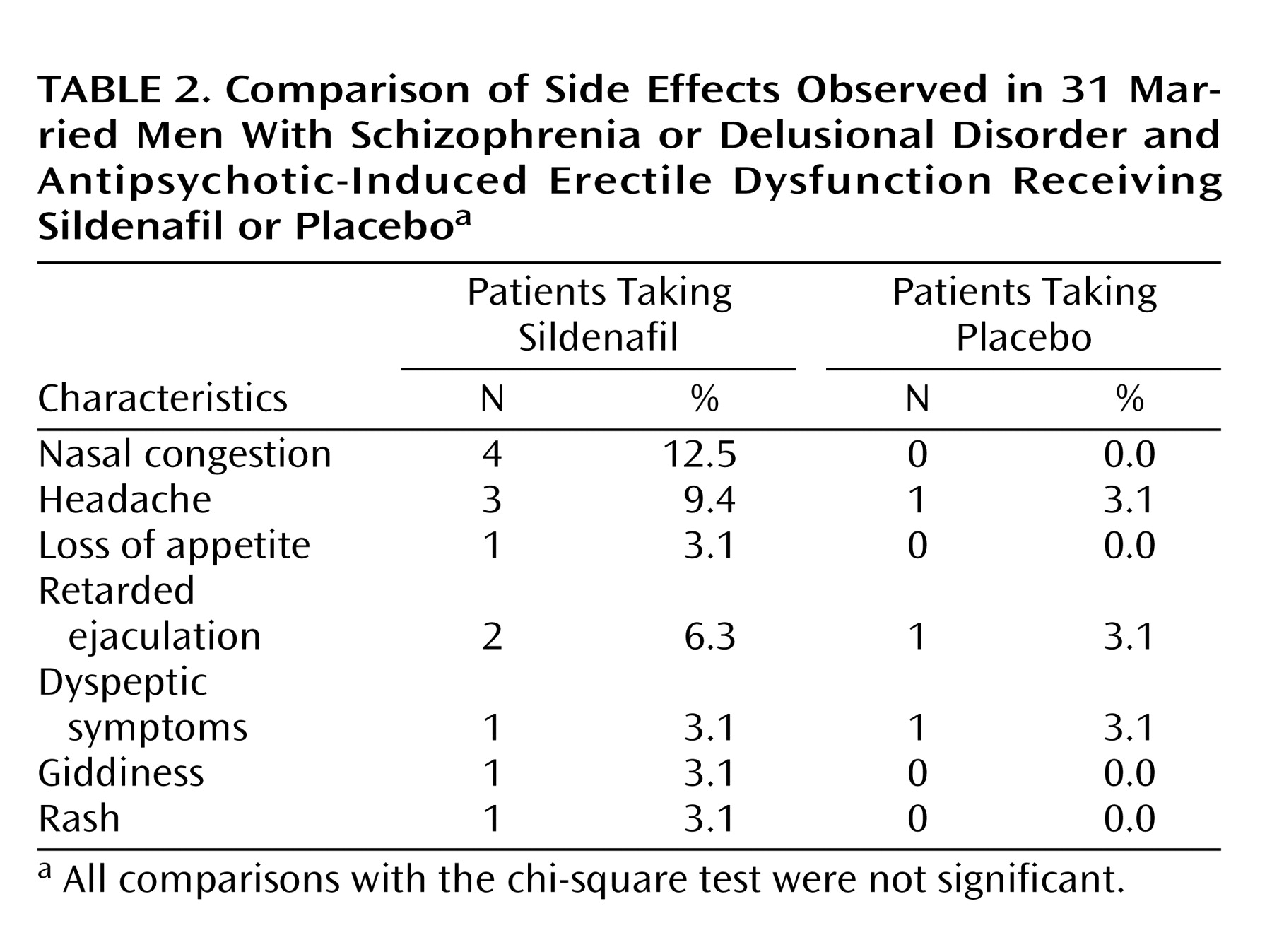
Sildenafil was well tolerated by most patients (
Table 2). No patient discontinued the trial or dropped out of the study as a result of adverse drug reactions. The most common side effects were nasal stuffiness (N=4, 12.5%) and headache (N=3, 9.4%). No adverse drug interactions were reported.
Efficacy in Subjects With Raised Serum Prolactin Levels
A similar analysis was done in a subgroup of subjects with elevated serum prolactin levels. The standardized normal range of serum prolactin for men in our laboratory is 5–17 ng/ml. The analysis among those with high serum prolactin (N=22, 68.8%) with the Wilcoxon matched-pair signed rank test revealed significant differences between the sildenafil and placebo treatments for the number of grade 3 or 4 erections (z=–3.51, p<0.001), the number of satisfactory erections (z=–3.44, p<0.001), and the duration of erections (z=–2.61, p<0.05). The results show that sildenafil is also effective in the subgroup of subjects with an elevated serum prolactin level.
Discussion
Erectile dysfunction is defined as the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual activity
(17). It is a common condition in middle-age and older men and frequently occurs in association with various illnesses or medications. Although a psychotic illness itself can cause sexual difficulties
(18), antipsychotics have been reported in case studies to be associated with sexual dysfunction in a significant proportion of patients
(19), with about 30% of patients taking newer atypical antipsychotic agents and around 60% taking conventional drugs reporting such problems
(1,
4,
19–24). The traditional management of sexual side effects involves using psychological techniques or oral drugs that are not of proven efficacy. Although sexual side effects are distressing to the patient, little systematic research with randomized trial designs has been performed in this area
(25).
This study employed a randomized, double-blind, placebo-controlled, flexible-dose, two-way crossover design to assess the efficacy and tolerability of sildenafil citrate in the treatment of antipsychotic-induced erectile dysfunction. Analysis of data from all patients who completed the trial revealed that sildenafil was associated with significant improvements in sexual function on a variety of parameters. The number of grade 3 or 4 erections increased from baseline in both treatments; however, the number was significantly higher during sildenafil treatment, especially with higher doses. The absence of significant period and treatment-period interactions suggests that the results of the trial are valid. An analysis of the subgroup with raised serum prolactin levels also revealed similar results. These findings are similar to those reported from earlier studies with sildenafil in erectile dysfunction of mixed etiology as well as organic causation, such as diabetes and spinal cord injury
(26,
27). These are also in concordance with earlier reports that sildenafil improves the quality of erections as well as the satisfaction associated with sexual intercourse
(7).
Sildenafil was well tolerated with no discontinuations due to adverse events. The majority of adverse events were mild or moderate. The frequencies of adverse events were similar to those from previous published trials with sildenafil
(7). There were no differences in the adverse events between the two doses. There were no major adverse drug interactions noted during the study period, and it can be assumed that sildenafil can be safely combined with antipsychotics. However, more evidence of this effect with higher doses of sildenafil is warranted.
Several limitations merit acknowledgment. Organic causes of erectile dysfunction were ruled out from a detailed history and by physical examination by the research psychiatrist. Basic laboratory investigations and blood sugar levels were normal. The study was performed in the natural environment, requiring a reliance on the study participants’ own reports of efficacy. Although of great value, self-reported sexual function and symptoms might not be accurate in all settings. Although a variable-dose schedule was used, we restricted the upper limit of the dose of sildenafil to 50 mg, and this could have caused nonresponse in a few patients. Clinical experience with sildenafil in erectile dysfunction in this population has shown that these patients required lower doses than what is used in the West. Hence, it was decided to use 25 mg and 50 mg for the trial. The group was also restricted by the inclusion of married subjects only in conformity with regional ethical standards. Other populations also need to be studied for efficacy.