Since the therapeutic effects of antipsychotics were first discovered, it has been clear that they produce undesirable as well as target effects, some of which are difficult to distinguish from the negative symptoms of schizophrenia
(1–
5). Motor side effects can mimic negative symptoms, as well as nonmotor type effects, including indifference, apathy, and avolition. These effects have been more frequently described in patient self-reports than in observations made by clinicians. These drug-induced side effects are difficult to distinguish from primary or other sources of secondary negative symptoms
(6,
7).
Both first- and second-generation antipsychotics reduce negative symptom ratings in schizophrenia. Several studies
(8,
9) have reported higher efficacy in negative symptoms with second-generation antipsychotics than with haloperidol; however, the differential effect may be lost when low doses of haloperidol are used
(10).
The effect of antipsychotics on primary negative symptoms is more controversial. Two general approaches have been applied to address this issue. On one hand, primary negative symptoms have been defined as observer-rated negative symptoms that cannot be explained by positive symptoms, depressive symptoms, or antipsychotic-produced motor extrapyramidal symptoms. Statistical techniques have evolved from simpler, correlational, and covariant strategies
(11–
13) to more sophisticated ones, such as path analysis
(8,
9,
14). The unexplained variance is interpreted as a direct treatment effect on primary negative symptoms. However, there are also other sources of secondary negative symptoms, and nonmotor effects of antipsychotics could be considered primary negative symptoms. Therefore, to deal with this, some investigators use a clinically defined and rated condition called the “deficit syndrome.” To be categorized as such, all known sources of secondary negative symptoms have to be ruled out, and the negative symptoms have to be long-lasting, with a longitudinal assessment
(15–
17).
Negative symptoms are easy to measure in schizophrenia patients but difficult to attribute to a precise source. To clarify these ambiguities, we studied healthy subjects free of any psychiatric symptoms. We assessed all negative symptoms in healthy subjects in response to single doses of haloperidol or risperidone in a double-blind, placebo-controlled trial. We quantified the subjective experiences of treated individuals as well as the observed effects. According to our hypothesis, haloperidol would cause the most negative symptoms as measured by observer-rated and subjective measures and placebo the least.
Method
Subjects
The subjects were recruited from advertisements placed in the Hospital Universitario 12 de Octubre in Madrid and met the following criteria: ages 18 to 60 years, no psychiatric disorder according to the Structured Clinical Interview for DSM-IV for normal volunteers, not receiving psychotropic drugs, no drug or alcohol abuse or dependence (except for nicotine), and no other relevant medical condition. The hospital’s ethical committee for research with humans approved the research protocol. Written informed consent was obtained from all subjects after we fully explained the procedure.
Design
A within-subject design was carried out with haloperidol and risperidone. Each participant was interviewed and rated four times. The first interview established baseline variables (with handwriting and a videotaped interview). The subsequent three interviews were identical and were performed after double-blind random assignment of the sequence with which placebo, haloperidol, and risperidone were to be administered.
Procedure
Because of ethical considerations, only single low doses of the substances were administered in liquid formulations of placebo, haloperidol (5 mg), or risperidone (2.5 mg). Water and lemon extract were added to the substances to ensure double-blind conditions
(18). The 2.5-mg dose of risperidone was chosen as the equivalent of 5 mg of haloperidol
(19,
20).
The baseline assessments were repeated at two different time points. The first evaluation (clinician-rated scales) was made at the time the substances reached their highest plasma level (T
max). T
max for haloperidol is 1.7–6.1 hours
(21); it is 0.8 hour (SD=0.3) for risperidone, and 3.2 hours (SD=1.5) for its active metabolite 9-hydroxyrisperidone
(22). Therefore, ratings were performed between 3 and 4 hours after administration for the first assessment. The second assessment (self-rated scales) was performed 24 hours after administration and assessed cumulative subjective experiences over the previous 24 hours. The minimum washout period between administrations was 48 hours based on half-lives of haloperidol (14.5–36.7 hours)
(21), risperidone (mean=2.8 hours, SD=0.5), and 9-hydroxyrisperidone (mean=20.5 hours, SD=2.9)
(22).
Motor Signs
Parkinsonism and akathisia were evaluated with standardized scales: the Simpson-Angus Rating Scale
(23) and the Barnes Rating Scale for Drug-Induced Akathisia
(24), respectively. Handwriting was evaluated as another variable of motor outcome. Decreased handwriting area has been described as a more precise way to rate motor extrapyramidal symptoms, in correlation with dopamine D
2 receptor occupancy
(25).
Observer-Rated Negative Symptoms
Negative symptoms were assessed with 1) the Brief Psychiatric Rating Scale (BPRS)
(26) negative symptoms subscale and 2) the Scale for the Assessment of Negative Symptoms (SANS)
(27) alogia and affect flattening items. Alogia and affective flattening were selected from the other items because they appear in several factor analyses in the negative syndrome, and they do not require a full week of observation. Instead of referring to illness during the interview, because the participants did not have schizophrenia, they were asked to talk about their most recent holidays. Both of the raters were psychiatrists (J.F.A. and J.S.). Interrater reliability scores (intercorrelation coefficients) for the two raters on these scales were between 0.80 and 0.92 for each BPRS item, each of the two SANS items, and the Simpson-Angus Rating Scale and Barnes Rating Scale for Drug-Induced Akathisia total scores. All four interviews (baseline and after administration of the placebo and the two drugs) were videotaped, and the raters made a consensus rating after watching the taped interviews.
Subjective Negative Symptoms
The Subjective Deficit Syndrome Scale was applied
(28). This scale asks subjects to rate, from 0 to 4, aspects regarding lack of energy, blunted affect, and difficulty in or altered thinking, including, “Do you tire easily (complaints about excessive fatigue)?” “Have you lost the ability to feel emotions (apathy)?” “Do you have problems concentrating (subjective problems with concentration)?” An analog scale
(29) of subjective negative symptoms was also developed based on Huber’s basic symptoms
(30) (the scale is available upon request from the first author). Both scales were self-reported.
Drowsiness
This concept is defined on the basis of Lewander’s consideration of sedation, which distinguishes between a more general somnolence effect versus a more lethargic effect
(4). An analog scale
(29) was developed to measure the sedative or somnolent effect. These scales present a line with two ends; each end expresses a specific maximum and minimum for the variable being evaluated. The subject is asked to make a mark on the line representing how he or she feels. (The scale is available upon request from the first author.) This variable was considered a possible confounding factor in the rating of observer-rated and self-rated negative symptoms.
Data Analysis
A balanced crossover experimental design was used, with the three treatments and six possible sequences of administration to address the issue of treatment order. Five individuals were randomly included in each sequence.
Data were analyzed as usual in this type of design with repeated-measures analysis of variance (ANOVA) and treatment and order of administration as fixed factors. The overall null hypothesis (placebo=haloperidol=risperidone) was tested for each outcome measured by using a continuous variable. If this null hypothesis was rejected, pairwise tests of the differences between treatments (haloperidol versus placebo, risperidone versus placebo, and risperidone versus haloperidol) were then performed with Student’s t test with Bonferroni correction. When the outcome was measured with a noncontinuous variable (Barnes Rating Scale for Drug-Induced Akathisia, the Simpson-Angus Rating Scale, or the SANS), only pairwise comparisons were made because the number of observations was insufficient to guarantee the requirements of the overall test.
A significance level of 0.05 was established. Some outcomes that might be affected by drowsiness, specifically observer- and self-rated negative symptoms, were included in the model as covariates. To rule out any possible confounding effect of other epidemiological variables (gender, age, smoking status, level of education, or body mass index), we applied ANOVA and chi-square tests to be sure that they were not correlated with the sequence of administration.
Results
Thirty-two subjects entered the study. Thirty subjects completed it; one person dropped out voluntarily, whereas another was eliminated after suffering a hypotensive episode with risperidone. Of the completer group, 17 were women and 13 were men; the mean age was 32.4 years (SD=10.5, range=18–58). Ten of the completers were smokers. Mean education in years was 17.1 (SD=1.6, range=12–18). Body mass index was 24.7 kg/m2 (SD=7.1, range=18.8–38.1). The variables were homogeneously distributed in the different groups and randomly assigned to the different sequences of drug administration. No significant differences attributable to the order of administration were detected for any of the variables.
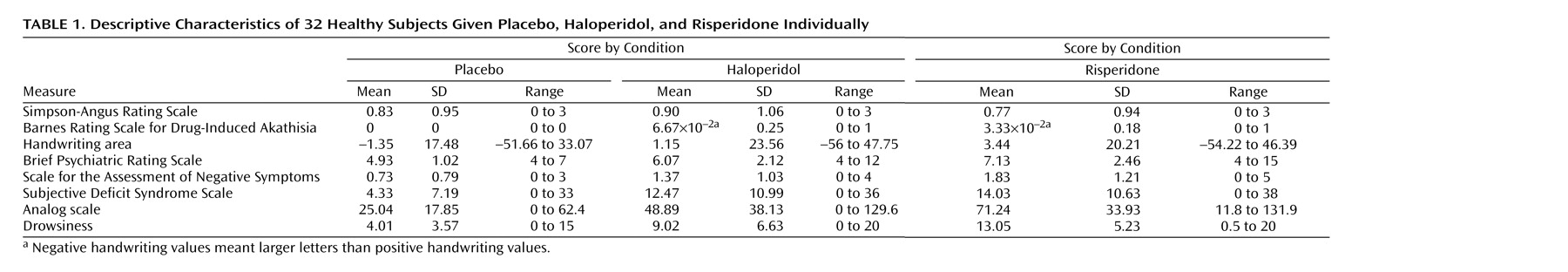
Descriptive characteristics and differences between treatments, before and after control for drowsiness, are shown in
Table 1 and
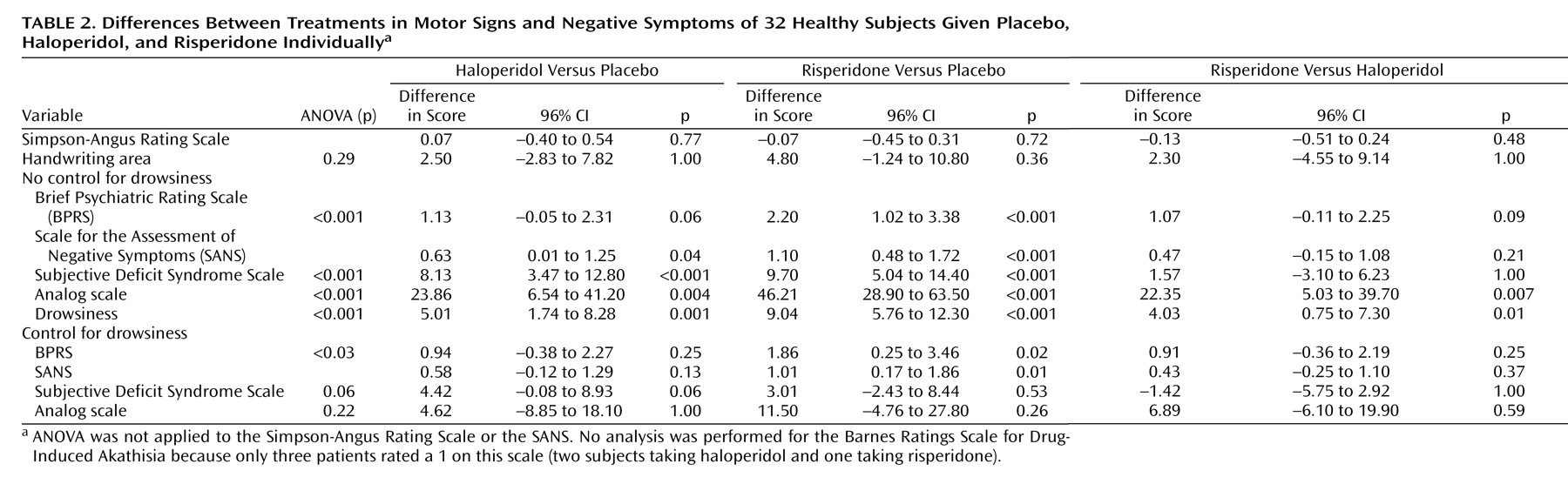
Table 2. As for motor symptoms, one subject reported acute buccolingual dystonia 20 hours after receiving haloperidol. No significant differences were detected between drugs for the Simpson-Angus Rating Scale score or the handwriting area. No analysis was performed for the Barnes Rating Scale for Drug-Induced Akathisia because only three patients rated a 1 on this scale (two subjects taking haloperidol and one taking risperidone). The participants taking risperidone scored significantly higher on the BPRS negative symptoms subscale than those receiving placebo; likewise, the individuals receiving haloperidol or risperidone scored significantly higher on the SANS items versus those receiving placebo. The participants taking haloperidol or risperidone scored significantly higher on the two self-rated scales than those receiving placebo (
Table 1 and
Table 2). The subjects scored significantly higher on drowsiness after taking either haloperidol or risperidone versus those receiving placebo; in a comparison of the two drugs, they scored higher with risperidone than with haloperidol (
Table 1 and
Table 2). After control for drowsiness, only those taking risperidone scored significantly higher than those receiving placebo on the BPRS and SANS negative symptom items (
Table 2).
Discussion
The results of the current study indicate that single-dose administration of both haloperidol and risperidone produces negative symptoms in healthy volunteers, according to both clinician-rated and self-report scales. Neither of the drugs produced significant motor extrapyramidal symptoms, as measured by the Simpson-Angus Rating Scale or the handwriting area, based on the findings of this single-dose study. Negative symptoms were found in the absence of a detectable motor extrapyramidal component. In the case of risperidone, negative symptoms were also independent of a sedative effect.
Treatment-induced nonmotor negative symptoms are not taken into account in studies in which primary negative symptoms are defined as observer-rated negative symptoms that cannot be explained by positive, depressive, and motor symptoms
(8,
9,
14). Because our normal comparison subjects lacked any initial psychopathology, the drug-induced nature of these symptoms is clear. Thus, we can say that the symptoms are “nonmotor” negative symptoms secondary to antipsychotics. In the studies cited
(8,
9,
14), unexplained variance is interpreted as a direct treatment effect exerted on primary negative symptoms by different second-generation antipsychotics. In these studies, antipsychotic-induced “nonmotor” negative symptoms could have been considered primary negative symptoms. The estimates of effect size for the medication-induced negative symptoms found in the present study could be useful in evaluating findings from pharmacological trials of agents that target negative symptoms.
Some authors
(4) give definitions for unspecific sedation (drowsiness) and specific sedation (Rifkin’s akinesia, which we have called negative signs and symptoms secondary to antipsychotics) that clearly differentiate them conceptually. Nonetheless, we find it complicated to distinguish between them in this manner. In fact, in the present study, subjective negative symptoms scales could not clearly distinguish between drowsiness and negative signs and symptoms secondary to antipsychotics. The information provided by these scales must be questioned until they can distinguish more clearly between these two factors. This idea receives strong support from data from various authors and clinical descriptions. The point is clarified further by Laborit’s description:
The patients required less analgesics and, at the same time, had an unusual state of consciousness in that they were readily aroused but seemed unconcerned with the environmental situation.
(1)
and by an unprompted written report by one of our subjects:
I feel slow but not sleepy. During the interview I feel clumsy, and I want to finish as soon as possible (it’s difficult for me to explain what is happening to me).
Drowsiness was also used as a possible confounding factor in the rating of observer-rated negative symptoms scales (BPRS and SANS). Albeit to a lesser degree than the “subjective” scales in our study, these scales were also affected to the point of rendering insignificant the difference between haloperidol and placebo. However, the differences between risperidone and placebo remained significant. Drowsiness is hardly ever controlled in the statistical approaches used to demonstrate the effect of drugs on primary negative symptoms. Moreover, high doses of benzodiazepines can provoke drowsiness, and this factor might be confounded with observer-rated negative symptoms.
An interpretation of the differences between haloperidol and risperidone depends on their equivalent doses. Several studies have suggested that the doses chosen in the present study were equipotential. The 1997 APA guidelines
(19) indicated that a 1–2-mg dose of risperidone equals a 2-mg dose of haloperidol and that haloperidol may require doses double those of risperidone. Schotte et al.
(20) measured the occupancy of central neurotransmitter receptors by haloperidol and risperidone in an ex vivo quantitative autoradiography. Doses of 0.048 mg/kg of haloperidol and 0.11 mg/kg of risperidone were needed to induce 25% D
2 receptor occupancy in the caudate-putamen. However, other studies have supported equivalent dosing
(31).
Our study group consisted of healthy individuals receiving single doses of antipsychotics. The effect of antipsychotics in patients with schizophrenia may differ from that seen in this study with normal comparison subjects. The study was conducted with a single dose, and the effect of these antipsychotics on the variables assessed could change in chronic settings. Nevertheless, both a review of the literature and clinical experience support the finding that antipsychotics produce negative signs and symptoms secondary to antipsychotics, not only in healthy subjects as shown in the present study but also in schizophrenia patients in long-term treatment. A further limitation was that a benzodiazepine arm was not included, which would have made it possible to compare the purer sedative effect and the neuroleptic effect caused by the antipsychotics. Drowsiness and subjective negative symptoms were assessed by self-rated scales 24 hours after drug administration to detect all symptoms that appeared during the entire 24-hour period. However, the observer-rated negative symptoms and motor variables were measured after 3–4 hours. Correction for those variables with another variable evaluated at a later time point could be a potential confounding factor. Finally, the dose chosen might have favored risperidone versus haloperidol, because although some reports stated that risperidone is equivalent to haloperidol, we used half the dose of risperidone for comparison with haloperidol.