The relationship between schizophrenia and bipolar disorder has been controversial since the disorders were distinguished by Kraepelin a century ago. There is considerable overlap in clinical features and in genetic and epidemiological risk factors between the two illnesses, although schizophrenia is more consistently linked to indices of neurodevelopmental disturbance
(1). There is also some evidence that schizophrenia is characterized by more extensive brain structural deviations than are found in bipolar disorder. Meta-analyses performed by our group have demonstrated that schizophrenia is associated with enlarged lateral ventricles and reduced volume of the cerebrum, medial temporal lobes, lateral temporal cortex, and thalamus
(2), whereas the only consistent finding to emerge for bipolar disorder is lateral ventricular enlargement
(3). However, the morphometry of bipolar disorder is relatively underresearched and considerably heterogeneous. There is some evidence from studies that compared groups of patients with bipolar disorder and patients with schizophrenia with the identical comparison group that ventricular enlargement is also found in male patients with bipolar disorder
(4) and in psychotic bipolar disorder
(5) and that hippocampal volume deficits are specific to schizophrenia
(6).
Some of the morphometric deviations associated with schizophrenia have been reported in unaffected first-degree relatives of patients at high likelihood of carrying susceptibility genes for the illness, including lateral ventricular enlargement
(7), hippocampal volume reduction
(8), and deficits of the prefrontal cortex
(9), suggesting that these brain abnormalities are endophenotypic for schizophrenia. However, the results are inconsistent. We previously found that hippocampal volume was preserved in unaffected relatives of patients with schizophrenia
(10). Unaffected relatives of patients with bipolar disorder have been rarely studied. In an exploratory computational morphometry study linking variable genetic liability with regional brain structure in families multiply affected with schizophrenia or bipolar disorder, we reported different genetic effects on gray matter in the two conditions. Thus, genetically mediated gray matter deficits in prefrontal, thalamic, and lateral temporal regions were found in schizophrenia, whereas much less extensive and nonoverlapping gray matter deficits in the right anterior cingulate and the ventral striatum were linked to genetic risk for bipolar disorder
(11). On the other hand, genetic risk was linked to left frontal and temporal-parietal white matter deficits in both disorders
(11).
In the present study, we used hypothesis-based region-of-interest morphometry in a similar group to assess whether specific structures commonly found to deviate morphometrically in psychotic illness (the cerebrum, ventricles, and hippocampus) are abnormal in patients with schizophrenia and in a homogeneous group of patients with bipolar I disorder who had experienced psychotic symptoms (any delusions and/or hallucinations) during one or more affective episodes. We considered that such patients would be more likely to share neurobiological abnormalities with schizophrenia than nonpsychotic forms of bipolar disorder. We also assessed whether any morphometric deviations might reflect the impact of susceptibility genes for illness by virtue of their presence in unaffected first-degree relatives of the patients at high likelihood of carrying such genes.
Our specific hypotheses included the following:
1.
Patients with schizophrenia would have increased lateral and third ventricular volume and decreased hippocampal volume in relation to comparison subjects.
2.
Patients with bipolar disorder would have increased lateral ventricular volume but preserved hippocampal volume.
3.
Unaffected relatives of patients with schizophrenia would have increased ventricular volume in relation to comparison subjects, which would be most prominent in the relatives from multiply affected families.
4.
Unaffected relatives of patients with bipolar disorder would have increased ventricular volume.
5.
Unaffected relatives of patients with schizophrenia or bipolar disorder would have preserved hippocampal volume.
Method
Subjects
Families affected with schizophrenia or bipolar disorder were recruited through voluntary support groups or through direct psychiatric referral. In the case of schizophrenia, we increased the variation of probable genetic liability by recruiting families who were either “familial,” defined as the index patient having other first- and/or second-degree relatives affected with a psychotic disorder (23 families), or “nonfamilial,” defined as the index patient having no known relatives with a psychotic disorder (19 families). All families with bipolar disorder were “familial” (32 families). The comparison subjects were recruited from the community through advertisements in local newspapers or from staff and were group-matched to the combined group of patients and relatives on the basis of age, gender, and parental social class. None of the comparison subjects had a personal or family history of psychosis. A lifetime history of nonpsychotic psychiatric illness was not an exclusion factor for the comparison subjects because it was not for unaffected relatives.
All of the subjects were ages 16–69 years, and their first language was English. The subjects were excluded if they had experienced organic brain disease, head trauma resulting in loss of consciousness for more than 5 minutes, or DSM-IV substance or alcohol dependence in the 12 months before the assessment. The study was approved by the relevant local ethics committees. After complete description of the study to each subject, written informed consent was obtained.
Structural magnetic resonance imaging (MRI) brain scans were successfully obtained for 243 subjects, comprising 42 patients with schizophrenia or schizoaffective disorder (N=3), 57 of their unaffected first-degree relatives, 38 patients with bipolar I disorder, 52 of their unaffected first-degree relatives, and 54 healthy comparison subjects. The majority of these subjects were included in our computational morphometry study of multiply affected families with schizophrenia or bipolar disorder
(11); the additional subjects in the present study comprised single-proband families and comparison subjects. There was no overlap between the subjects included in the present study and those included in our previous region-of-interest studies of families affected with schizophrenia
(7,
10).
Clinical Assessments
Structured diagnostic interviews were performed on all subjects with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version
(12), and additional clinical information was collected to enable lifetime DSM-IV diagnoses to be made. For relatives not assessed directly, information regarding psychiatric diagnoses was obtained from the most reliable informants with the Family Interview for Genetic Studies
(13) and supplemented by medical notes where available. The patients were also assessed with the Positive and Negative Syndrome Scale (PANSS)
(14). The Schedule for Schizotypal Personalities
(15) was used to assess nonpsychotic relatives and comparison subjects for schizotypal traits.
MRI Acquisition and Processing
For each subject, a set of 1.5-mm-thick contiguous coronal T1-weighted MRI images extending through the entire brain was obtained by using a three-dimensional spoiled gradient recall echo sequence on a 1.5-T GE N/Vi Signa System scanner (General Electric, Milwaukee) and the following protocol: TR=13.1 msec, TI=450 msec, TE=5.8 msec, number of excitations=1, flip angle=20°, acquisition matrix=256×256×128.
Each MRI was rated blind to group affiliation by using MEASURE (version 0.8, Johns Hopkins University, Baltimore), an image analysis program that employs stereologically unbiased estimation of volume
(16). Before we made any measurements, we corrected for head tilt by aligning each brain along the anterior commissure-posterior commissure axis in the sagittal plane and along the interhemispheric fissure in the coronal and axial planes. The following regions were rated: cerebral volume, right and left lateral ventricles, third ventricle, right and left hippocampi. The boundaries and grid measurements used for each of these regions are described in detail elsewhere
(7,
10).
Interrater reliability estimates based on a random sample of 10 brains calculated with the intraclass correlation coefficient were: cerebrum, 0.91; left lateral ventricle, 0.99; right lateral ventricle, 0.99; third ventricle, 0.98; right hippocampus, 0.86; left hippocampus, 0.87. Intrarater reliability estimates based 10 brains rated at least 6 months apart were all between 0.91 and 0.99.
Data Analysis
Morphometric analyses were performed by using Stata version 8.2 (Stata Corp., College Station, Tex.). The data were analyzed with multivariate linear regression with robust variance estimators for clustered data to account for any intrafamilial correlation of volumetric measurements. Each subject group was compared to the comparison group in a single analysis for each brain structure with volume as the dependent variable and control added for age, gender, handedness, height, a quadratic function of age, and lifetime history of alcohol or substance abuse. Direct comparisons of patients with schizophrenia and patients with bipolar disorder were also performed. Height was included as an independent variable because it is a good predictor of general head size
(17) and is independent of any disease-specific processes. A quadratic function of age was included to model potential nonlinear effects on brain structure. Cerebral volume was additionally included as a covariate in the analyses of hippocampal volumes. The distributions of measurements for lateral and third ventricular volumes were skewed, and values were successfully logarithmically transformed before analyses (Kolmogorov-Smirnov test, p>0.20). All tests were two-tailed and used a 0.05 level of significance.
Results
Subject Characteristics
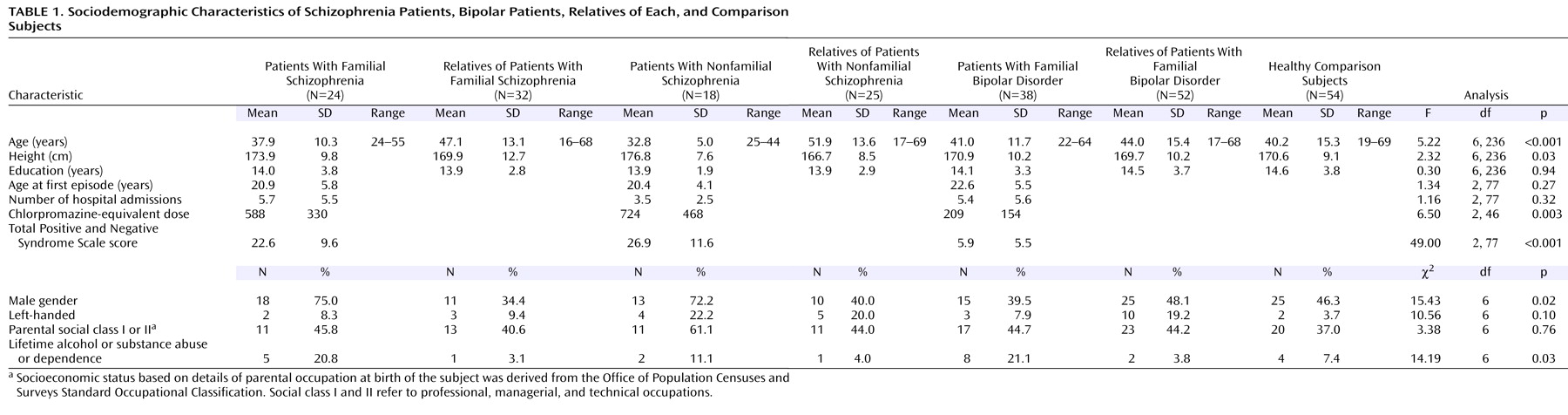
The sociodemographic and clinical details of the subjects are displayed in
Table 1. All subjects were Caucasian. There was a significant age difference between the groups. Bonferroni post hoc analysis revealed that the patients with nonfamilial schizophrenia were significantly younger than each relative group and that the relatives of nonfamilial patients with schizophrenia were older than each patient group and the comparison subjects. There was a larger proportion of men in the schizophrenia patient groups. Patients with nonfamilial schizophrenia were taller than their relatives, attributable to the higher proportion of men. There was a larger proportion of subjects with a lifetime history of alcohol or substance abuse among the patients. There were no significant differences between the subject groups in years of education, proportion of left-handers, or parental social class. The patients had a similar age at onset and number of hospitalizations. The patients with bipolar disorder had lower PANSS scores than the patients with schizophrenia.
Of the 57 unaffected relatives of the patients with schizophrenia, 13 had fulfilled criteria for another axis 1 disorder at some point in their lives: nine for major depressive disorder, two for panic disorder without agoraphobia, one for panic disorder with agoraphobia, and one for anxiety disorder not otherwise specified. Four relatives of patients with schizophrenia fulfilled criteria for schizotypal personality disorder. Of the 52 relatives of the patients with bipolar disorder, 10 had fulfilled criteria for major depressive disorder and one for panic disorder without agoraphobia. Five of the comparison subjects had fulfilled criteria for major depressive disorder at some point in their lives.
Forty-one patients from the schizophrenia group were receiving at least one antipsychotic medication, and one was taking a mood stabilizer. Of the patients with bipolar disorder, 33 were taking at least one mood stabilizer, one was taking olanzapine, and four were taking no medication. Ten patients with bipolar disorder were taking antipsychotic medications.
Regional Morphometry Findings
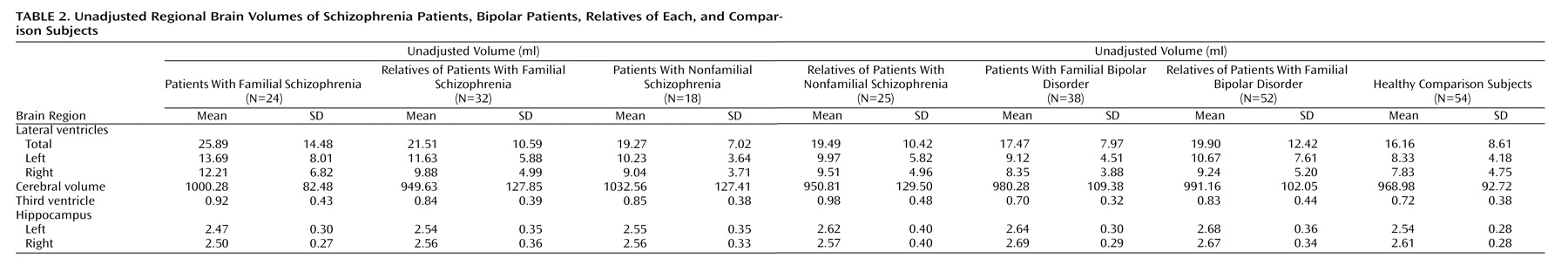
The mean unadjusted volumes of regional brain structure in each subject group are shown in
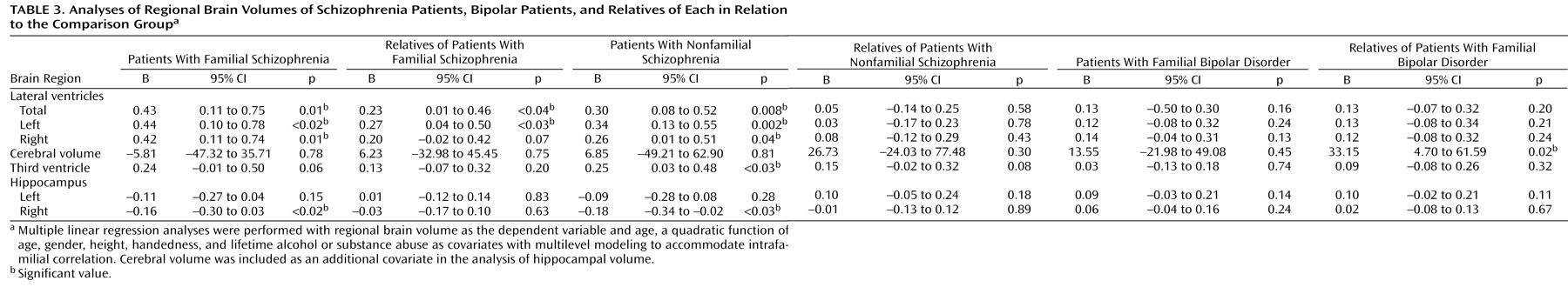
Table 2, and the results of the analyses are shown in
Table 3.
Lateral Ventricular Volume
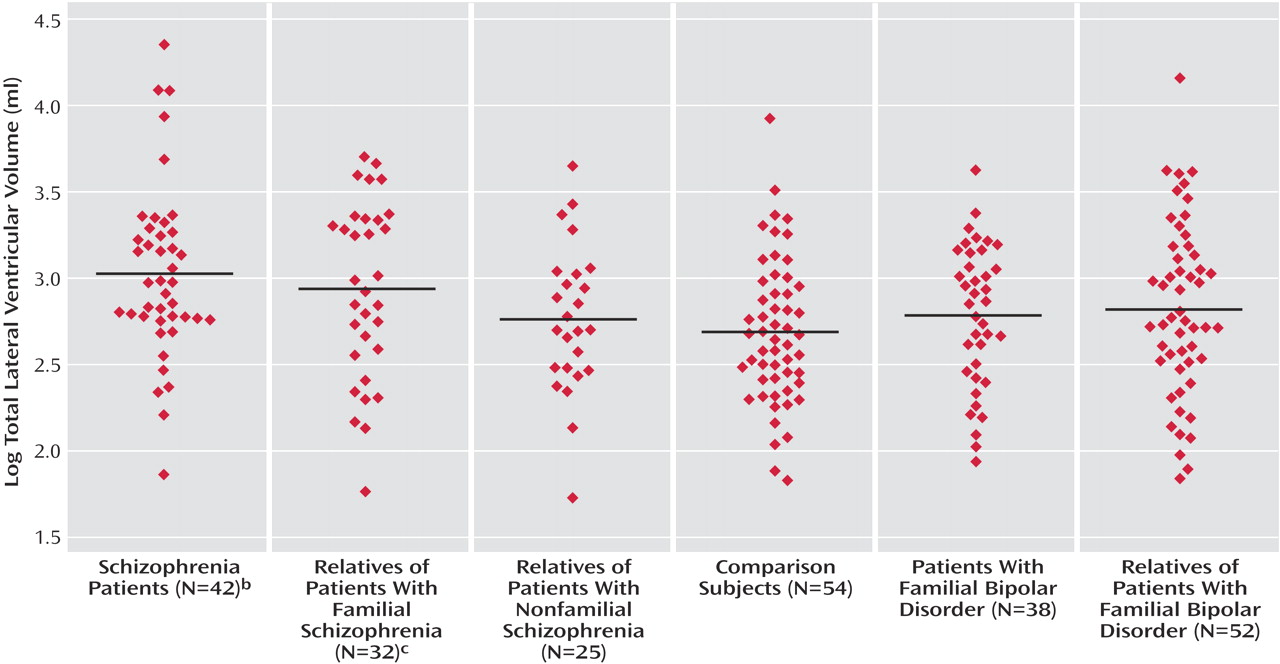
The patients with both familial and nonfamilial schizophrenia had significantly enlarged lateral ventricular volume bilaterally in relation to the comparison subjects. Total lateral ventricular volume did not differ between the two schizophrenia groups (B=0.24, p=0.27; 95% confidence interval [CI]=–0.20 to 0.69). The unaffected relatives of the patients with familial schizophrenia also had larger total lateral ventricular volume in relation to the comparison subjects that was more prominent on the left. Neither patients with bipolar disorder nor their unaffected relatives had lateral ventricular volumes that significantly differed from the comparison group. When they were directly compared, the patients with schizophrenia tended to have larger lateral ventricular volumes than the patients with bipolar disorder (B=0.23, p=0.07; 95% CI=–0.02 to 0.48). Because other psychiatric illnesses have been associated with ventricular enlargement, we repeated the analyses after excluding the 33 relatives and comparison subjects who had ever fulfilled criteria for a DSM-IV disorder as well as the three patients with schizoaffective disorder. The results were essentially unchanged; specifically, the unaffected relatives of the patients with familial schizophrenia continued to display larger total lateral ventricular volumes (B=0.26, p=0.056; 95% CI=–0.01 to 0.52) and left lateral ventricular volumes (B=0.29, p<0.05; 95% CI=0.01 to 0.56) in relation to the comparison subjects.
Figure 1 illustrates mean lateral ventricular volume in each group. There was strong evidence for a linear tendency between increasing genetic risk for schizophrenia and total lateral ventricular enlargement (B=0.13, p=0.001; 95% CI=0.06 to 0.21) when multiple linear regression analysis was repeated with a predictor variable of stratified likely genetic risk for schizophrenia—from comparison subjects to unaffected relatives, from singly affected families to unaffected relatives, and from multiply affected families to patients with schizophrenia.
Third Ventricular Volume
Third ventricular volume was significantly increased in the patients with nonfamilial schizophrenia, and the patients with familial schizophrenia showed a strong tendency for the same finding. The combined group of patents with schizophrenia had significantly larger third ventricular volumes than the comparison subjects (B=0.25, p=0.01; 95% CI=0.05 to 0.44). No other subject group differed significantly from the comparison group. The patients with schizophrenia had significantly larger third ventricular volume than the patients with bipolar disorder (B=0.22, p<0.05; 95% CI=0.01 to 0.44).
Cerebral Volume
Cerebral volume did not differ significantly between any patient group and the comparison group; however, the relatives of the patients with bipolar disorder had a slightly larger cerebral volume than the comparison subjects. There were no significant differences between the patients with schizophrenia and the patients with bipolar disorder (B=–10.56, p=0.63; 95% CI=–54.20 to 33.08).
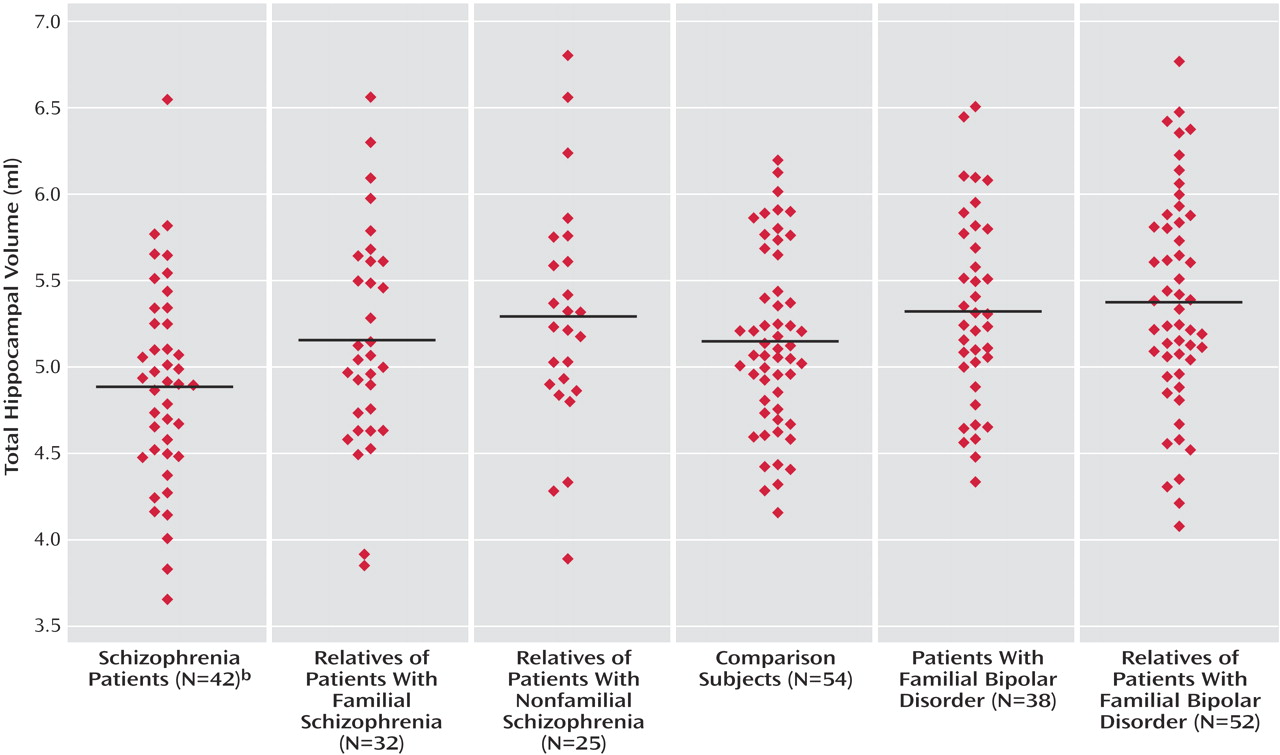
Hippocampal Volume
The patients with both familial and nonfamilial schizophrenia had significantly reduced right hippocampal volume in relation to the comparison subjects, but the differences in left hippocampal volume did not reach significance. However, there was a single patient with familial schizophrenia who had an outlying residual value for that subgroup. When this individual was excluded, the patients with familial schizophrenia demonstrated significantly reduced left hippocampal volume (B=–0.15, p<0.04; 95% CI=–0.28 to –0.01). Furthermore, the combined schizophrenia patient group displayed significantly reduced left hippocampal volume in relation to the comparison group (B=–0.13, p=0.04; 95% CI=–0.25 to –0.01).
The hippocampal volume measurements of each other subject group were similar to the comparison subjects. The patients with schizophrenia had smaller left hippocampal volumes (B=–0.19, p=0.02; 95% CI=–0.34 to –0.03) and right hippocampal volumes (B=–0.18, p<0.02, 95% CI=–0.31 to –0.04) than the patients with bipolar disorder.
Figure 2 demonstrates mean total hippocampal volumes for each subject group. After we excluded the 33 relatives and comparison subjects who had ever fulfilled criteria for a DSM-IV psychiatric disorder, none of the unaffected relative groups differed from the comparison group.
Gender Interactions
There were no significant group-by-gender interactions for any of the brain structures examined, indicating that the findings of the initial analyses were not mediated by subjects of one gender (results are available from the first author). When we controlled for age, there were no significant correlations between duration of illness and any of the volumetric measurements for the patients with schizophrenia or the patients with bipolar disorder.
Discussion
Morphometric Deviations of Schizophrenia and Bipolar Disorder
This large study clearly demonstrates the morphometric abnormalities most commonly associated with schizophrenia, i.e., lateral and third ventricular enlargement and hippocampal volume reduction
(2,
18). Thus, our first hypothesis was confirmed. However, we found that none of these deviations was shared with psychotic bipolar disorder. Thus, our second hypothesis was partially supported in that hippocampal volumes were preserved in bipolar disorder but partly negated in that ventricular volumes did not differ from those of the comparison subjects. Direct comparison of the two patient groups and the use of an identical comparison group underlined the specificity of the changes in schizophrenia. These region-of-interest results are consistent with a computational morphometry study of a similar group in which schizophrenia, but not psychotic bipolar disorder, was associated with gray matter deficits in the bilateral hippocampus and the thalamus (which borders most of the third ventricle) as well as other regions within the frontal-temporal cortex and cerebellum
(19).
Some previous structural imaging studies have reported lateral ventricular enlargement in bipolar disorder
(3). A recent study reported that ventricular enlargement characterized schizophrenia and psychotic bipolar disorder but not nonpsychotic bipolar disorder
(5). Methodological differences between this study and our own may account for the discrepancy in our findings as well as group variation. Despite the group size and the relative clinical homogeneity of our bipolar disorder patients, a type II error in the present study is possible. Of interest, the factor increase of 0.14 for the log-transformed right lateral ventricular volume comparison of bipolar disorder with the comparison subjects was precisely the same as the extent of enlargement (14%) of this structure identified by our recent meta-analysis
(3); however, the particularly large variance of ventricular volume (when compared with other regional brain measurements) rendered the comparison statistically nonsignificant.
Our findings indicate that hippocampal volume is preserved in familial psychotic bipolar disorder subjects. This is consistent with the majority of case-control studies of the wider bipolar disorder spectrum
(3) and contrasts with the hippocampal volume deficit characteristic of both schizophrenia
(2) and unipolar depression
(20). Previous studies that compared hippocampal volume in patients with schizophrenia and bipolar disorder (or “affective psychosis”) with each other or the same comparison group have mostly found that volume deficits were specific to schizophrenia
(6,
21,
22), although hippocampal deficits in both disorders have been reported
(23).
Morphometric Endophenotypes in Schizophrenia and Bipolar Disorder
Our third hypothesis was confirmed in that unaffected relatives of patients with familial schizophrenia showed lateral ventricular enlargement, thus supporting the latter as a potential endophenotype of schizophrenia. This is consistent with a previous study from our group of an independent group of subjects in which relatives most likely to carry susceptibility genes for schizophrenia had significantly enlarged lateral ventricles
(7). In contrast to our previous study
(7), there were no significant interactions with gender. We did not find evidence to support third ventricular volume enlargement as an endophenotype for schizophrenia in the present study, in contrast to some other studies of unaffected first-degree relatives
(7,
24).
This is the first regional morphometry study of unaffected first-degree relatives of patients with bipolar disorder, to our knowledge. We found no evidence for ventricular volume enlargement, thus we failed to support our fourth hypothesis.
The slight increase in cerebral volume in the relatives of patients with bipolar disorder in relation to the comparison group was unexpected but has some precedence in the neuropsychological literature, in which such relatives were reported to perform better than relatives of patients with schizophrenia and comparison subjects on measures of IQ
(25) and premorbid IQ
(26), although deficits in specific domains, such as declarative memory and executive function, are also reported. Given the unexpected direction of this finding and the number of statistical tests performed, this result is tentative and warrants replication.
The study confirmed our fifth hypothesis in that it provided no evidence that hippocampal volume reduction is endophenotypic for schizophrenia or for bipolar disorder. This is consistent with our computational morphometry study, which did not find any association between increasing genetic liability and medial-temporal lobe deficits
(11) as well as with our previous study of an independent group of families
(10) and with some other studies of relatives of patients with schizophrenia
(27,
28). However, it contrasts with other volumetric studies of the hippocampus
(8,
29,
30) in relatives of patients with schizophrenia or adolescents/young adults at a high risk of developing schizophrenia
(24). On top of these conflicting findings, the utility of hippocampal volume as an endophenotype is undermined by its relatively low heritability (0.40) compared to other brain morphometric measures
(31), which is consistent with evidence from twin studies of schizophrenia for nongenetic factors affecting hippocampal volume
(30,
32) and the susceptibility of the hippocampus to environmental risk factors, such as obstetric complications
(10,
29,
32,
33) and stress-induced glucocorticoid excess
(34). The preservation of hippocampal volume in unaffected relatives of bipolar disorder patients as well as the patients themselves provides further evidence that this structure is not affected by the genetic risk for bipolar disorder as might be hypothesized if, for example, genetically mediated hippocampal deficits were associated with bipolar disorder but reversed in patient groups because of the neurotrophic effects of treatment with mood stabilizers.
Limitations
In addition to the issue of type II error already discussed, our study has some further limitations. The subgroups were not all well matched for age and gender. This was inevitable for age given the study design because the parents were included in the relative groups. This imprecise matching was compensated for by recruiting a comparison group that spanned the age range in both genders and thus allowed tighter control for these confounders. Given the wide age range, a quadratic term was included to control for potential nonlinear effects of age on brain structure. The analyses were also repeated on subjects ages 50 and younger, and the findings were essentially unchanged (results available from first author), indicating that they were not solely driven by older individuals.
Although the majority of the unaffected relatives had lived through the major risk period for psychosis, some younger relatives were included, and a proportion (∼10%) of these are likely to develop illness. Because these account for a small a fraction of the relative group, any brain abnormalities associated with such individuals who might already be on a trajectory toward psychotic illness are unlikely to affect the results.
Given the group size and the methodology, a limited number of structures were chosen for measurement, and other structures implicated by our computational morphometry study
(11), such as the prefrontal cortex, anterior cingulate, thalamus, and regional white matter, may prove more fruitful areas to seek morphometric endophenotypes of psychotic illness.
The utility of lateral ventricular volume as an endophenotype for schizophrenia must be considered in the light of two issues. First, the questionable heritability of the volume of this structure shows some normal twin studies reporting high
(35) and others reporting low
(36) heritability estimates. Further twin studies are required to clarify this issue. Second, the nonspecificity of this finding is in light of evidence for an association between obstetric hazards and lateral ventricular enlargement, both in patients with schizophrenia
(7,
32) and in nonpsychotic individuals
(33). However, known major environmental risk factors can be either covaried for or the subjects affected can be excluded from studies seeking susceptibility genes for ventricular enlargement.
Conclusions
The present study provides morphometric support for the classic Kraepelinian distinction between these two major psychotic disorders, whereby lateral and third ventricular enlargement and hippocampal volume reduction characterize schizophrenia but not psychotic bipolar disorder. We provide evidence that lateral ventricular enlargement is a marker of genetic liability for schizophrenia and demonstrate that genetic liability for bipolar disorder is not reflected in abnormalities of ventricular or hippocampal volume.