Dopamine D
2 receptor blockade is a property of all known antipsychotics
(1), yet a predictive relationship between variation in the dopamine D
2 receptor gene (
DRD2) and medication response has not been clearly established
(2). One possible reason is that long-term treatment with antipsychotics causes substantial changes (specifically, up-regulation) in dopamine receptor physiology
(3). Thus, treatment studies of patients experiencing their first episode of schizophrenia may be methodologically advantageous in examining effects of this gene. To date, there has only been one pharmacogenetic study reported with a subject group consisting entirely of first-episode patients
(4), which found that genes coding for the D
3 and serotonin receptor subtypes were related to symptom reduction after 10 weeks of naturalistic treatment with a variety of antipsychotics. However, no association to treatment response was found with the only
DRD2 polymorphism examined (Taq1A). It is important to note that Taq1A is actually located downstream from
DRD2 itself
(5), and its significance to gene expression is unclear.
On the other hand, single nucleotide polymorphisms (SNPs) in the 5′ promoter region are likely candidates for alteration of transcriptional activity
(6). Two
DRD2 promoter region SNPs have been identified: substitution of guanine for adenine at position –241 (A-241G) and a deletion (versus insertion) of cytosine at position –141 (–141C Ins/Del). –141C Ins/Del has been demonstrated to alter gene expression in vitro
(6), and one imaging study in healthy volunteers showed increased striatal receptor density in Del carriers
(7). While one pharmacogenetic study of this polymorphism has indicated reduced response to chlorpromazine in Del carriers
(8), other studies have shown inconsistent results
(2). No prior pharmacogenetic studies of this polymorphism have utilized exclusively first-episode patients. Further, no published studies of –141C Ins/Del have examined response to first-line atypical agents, time course of response, or effects of the A-241G polymorphism. The present study was designed to address these limitations in the context of a randomized controlled trial of risperidone and olanzapine.
Method
Subjects were a subset of participants in a clinical trial for patients with first-episode schizophrenia, schizoaffective disorder, or schizophreniform disorder, comprising the first 61 subjects (45 men and 16 women; mean age=24 years, SD=5, range=16–38) who provided written informed consent for both the trial and genetic analyses. Most subjects (79%, N=48) were antipsychotic-naive; none had more than 12 weeks of previous exposure. Subjects were randomly assigned to 16 weeks of treatment with olanzapine (2.5–20 mg/day, N=28) or risperidone (1–6 mg, N=33).
Raters blind to treatment condition and DRD2 genotype conducted weekly assessments during the first 4 weeks, then biweekly assessments. Response criteria were stringent, requiring the absence of delusions, hallucinations, or substantial thought disorder. A rating of 3 (mild) or less, as rated with the Schedule for Affective Disorders and Schizophrenia—Change Version, was needed on these items: severity of delusions, severity of hallucinations, impaired understandability, derailment, illogical thinking, and bizarre behavior. In addition, a rating of very much or much improved on the Clinical Global Impression improvement scale was required. These criteria were required to be sustained for two consecutive ratings.
Patients were genotyped for A-241G and –141C Ins/Del by 5′-exonuclease fluorescence assay. Genotype frequencies were in Hardy-Weinberg equilibrium (p>0.39). For data analysis, patients were dichotomized as homozygous for the wild type allele or as carriers of the rare allele for each SNP (for A–241G: minor allele frequency=12.3%; for –141C Ins/Del: minor allele frequency=32.8%). Genotype groups (A/A versus G carriers; Ins/Ins versus Del carriers) did not differ in terms of sex, diagnosis, or previous treatment (all p>0.42). Differences between groups in time to initiation of sustained response were examined by using Kaplan-Meier curves. Statistical significance for the two main comparisons was determined using the false discovery rate method (0.05 level).
Results
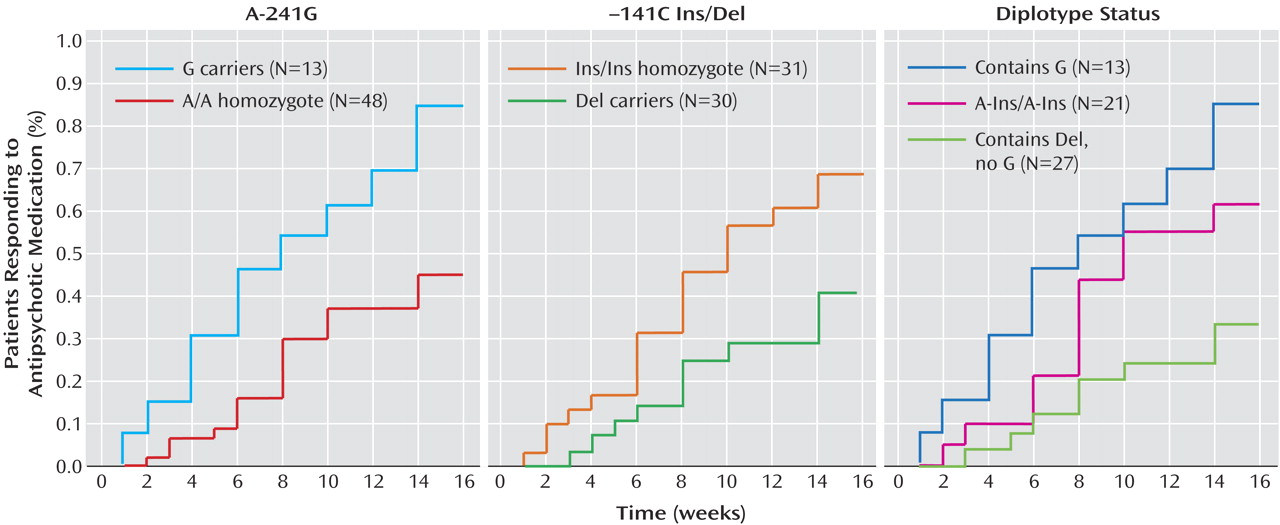
For each SNP, statistically significant differences were found in time to response to antipsychotic treatment. Carriers of the –241G allele showed significantly faster time until response compared with A/A homozygotes (log-rank=8.40, df=1, p<0.004) (
Figure 1). Carriers of the –141C Del allele showed significantly longer time to respond relative to Ins/Ins homozygotes (log-rank=5.03, df=1, p<0.03) (
Figure 1).
The two SNPs were only weakly associated (r
2=0.068). Observed diplotype frequencies were as follows: G-Ins/G-Ins (3.3%); A-Ins/G-Ins (13.1%); A-Ins/G-Del or G-Ins/A-Del (i.e., double heterozygotes, 4.9%); A-Ins/A-Ins (34.4%); A-Ins/A-Del (27.9%); A-Del/A-Del (16.4%). Subjects possessing at least one G allele at A-241G in their diplotype (N=13) showed relatively high rates of sustained response (83% met criteria within 16 weeks). Subjects with the A-Ins/A-Ins diplotype (N=21) were intermediate in their response rate (52%), and subjects whose diplotype contained at least one copy of the Del allele, but zero copies of G (N=27) were least likely to respond (30%) (χ
2=10.7, df=2, p=0.002). Kaplan-Meier survival analysis performed on these three groups was significant (log-rank=11.38, df=2, p<0.004) (
Figure 1). Pairwise comparisons indicated a significant difference between the first and third groups (log-rank=11.29, df=1, p=0.0008). Subjects with the A-Ins/A-Ins diplotype tended to have a faster response relative to the group containing at least one Del allele but no G allele in their diplotype (log-rank=3.70, df=1, p=0.054), but those with the A-Ins/A-Ins diplotype did not significantly differ from subjects whose diplotype contained G (log-rank=1.97, df=1, p=0.16).
Because of ethnic heterogeneity within the group (41% African American; 28% Caucasian (European); 18% Hispanic; 5% Asian; 8% other), the potential that population stratification influenced our results was addressed in two ways. First, there were no significant differences between ethnic groups on time until response (for all five groups, p=0.114; for African Americans compared with all others, p=0.204). Second, pharmacogenetic analyses were conducted for African American subjects (the largest single group) alone. Results remained significant for –241 G carriers (log-rank=16.41, df=1, p=0.0001) and approached significance for –141C Del carriers (log-rank=3.27, df=1, p=0.07). Diplotype analysis remained significant (log-rank=17.07, df=2, p=0.0002).
Discussion
To our knowledge, this is the first report to detect a significant relationship between
DRD2 genetic variation and treatment response in first-episode schizophrenia patients. While the association between
DRD2 promoter region variation and the presence of schizophrenia remains controversial
(9), such an association is not essential to the phenotype of differential treatment response within a patient group. Given prior functional studies of
DRD2 promoter region variation
(6,
7), our findings suggest the possibility that the timing of clinical response may be related to variation in density or binding potentials of D
2 receptors. For example, if Del noncarriers have decreased striatal D
2 receptor density, antipsychotics may be more efficient at achieving therapeutic levels of blockade. Further studies are needed to clarify the mechanism of these effects. Because of the small study group size, replication is required to confirm their potential clinical utility.