Dysbindin is a conserved protein that binds alpha and beta dystrobrevin, components of the dystrophin glycoprotein complex. Although there is a paucity of data describing the functional role of dysbindin, dysbindin mRNA is widely expressed in the human brain
(1), and molecular studies have linked the dysbindin gene (DTNBP1) to schizophrenia.
Straub and colleagues
(2) initially identified a significant linkage peak on chromosome 6p22 in 270 Irish schizophrenia pedigrees, and subsequent work narrowed the location to DTNBP1
(3). Numerous studies
(4) have replicated this association, and our group identified a 6-locus haplotype (CTCTAC) that is significantly overrepresented in Caucasians with schizophrenia
(5).
In follow-up work, we have found that carriers of this haplotype perform significantly worse than noncarriers on a global neurocognitive measure sampling from the domains of attention, working memory, and executive functioning
(6). This association was present in schizophrenia and in healthy volunteers.
In schizophrenia, cognitive deficits are often accompanied by negative symptoms and may represent different manifestations of a common underlying pathology. Based on our data linking the CTCTAC haplotype to cognitive dysfunction, we hypothesized that it may also be linked to negative symptoms. Specifically, we hypothesized that CTCTAC carriers would demonstrate a history of more prominent negative symptoms than noncarriers.
Method
The study included 181 Caucasian men and women meeting criteria based on the Structured Clinical Interview for DSM-IV (SCID) for schizophrenia or schizoaffective disorder. All subjects provided written informed consent to a protocol approved by the Institutional Review Board of the North Shore-Long Island Jewish Health System. Subjects were recruited from The Zucker Hillside Hospital, a division of the North Shore-Long Island Jewish Health System, in Glen Oaks, N.Y. Some of the patients in the current study were also included in previous reports
(5,
6).
Each patient was assessed with the SCID administered by trained and reliable raters. Negative symptom ratings, reflecting a lifetime history of negative symptoms, included the three negative symptom items in the SCID: avolition, alogia, and affective flattening. These items are rated on a continuous scale on which 1=absent, 2=subthreshold, and 3=present. Genomic DNA was extracted from venous blood and genotyped as described previously
(5).
The SNPHAP program (Department of Medical Genetics, Cambridge Institute for Medical Research, Addenbrooke’s Hospital, Cambridge, U.K.) was used for estimating haplotype frequencies. All individual haplotypes that did not exceed the probability of 0.95 were excluded. Multivariate analysis of variance (MANOVA) was used to compare ratings of the three negative symptoms between carriers and noncarriers of the CTCTAC haplotype. This analysis was followed by post hoc t tests to evaluate group differences on individual ratings. Ratings for symptoms that differed by group were then pooled to create a single measure that reflected the full severity of negative symptoms in each patient. A univariate analysis of variance (ANOVA) and an analysis of covariance (ANCOVA), which covaried for global neurocognitive functioning
(6) and estimated premorbid IQ (as measured by the reading subtest of the Wide-Range Achievement Test, 3rd ed. [WRAT-3]), were then conducted to assess the relation between the CTCTAC haplotype and the overall negative symptom rating. The ANCOVA was conducted to demonstrate that dysbindin genotype contributes to the severity of negative symptoms independently of its effect on neurocognition.
Results
Twenty-six carriers of the CTCTAC haplotype were identified in the group of 181 patients. Four homozygotes were identified, but they were grouped with the heterozygotes because they did not significantly differ from heterozygotes. Thus, all analyses were conducted between two groups: carriers and noncarriers. There were no significant differences between carriers and noncarriers, respectively, in sex (23.08% versus 32.90% were women) (χ2=1.73, df=1, p<0.19), age (mean=40.65 years, SD=9.68, versus mean=38.74, SD=10.59) (t=0.36, df=179, p=0.72), age at onset of schizophrenia (mean=20.72, SD=5.13, versus mean=21.12, SD=6.04) (t=–0.34, df=172, p=0.73), duration of illness (mean=19.60 years, SD=11.02, versus mean=17.35 years, SD=10.47) (t=0.76, df=170, p=0.45), or Global Assessment of Functioning Scale (GAF) score (mean=37.46, SD=10.29, versus mean=37.97, SD=15.77) (t=–0.27, df=172, p<0.79). A multiple linear regression assessing the ability of these variables to predict the overall negative symptom rating was not significant (F=0.72, df=5, 99, p<0.61). Therefore, these variables were excluded from further analyses.
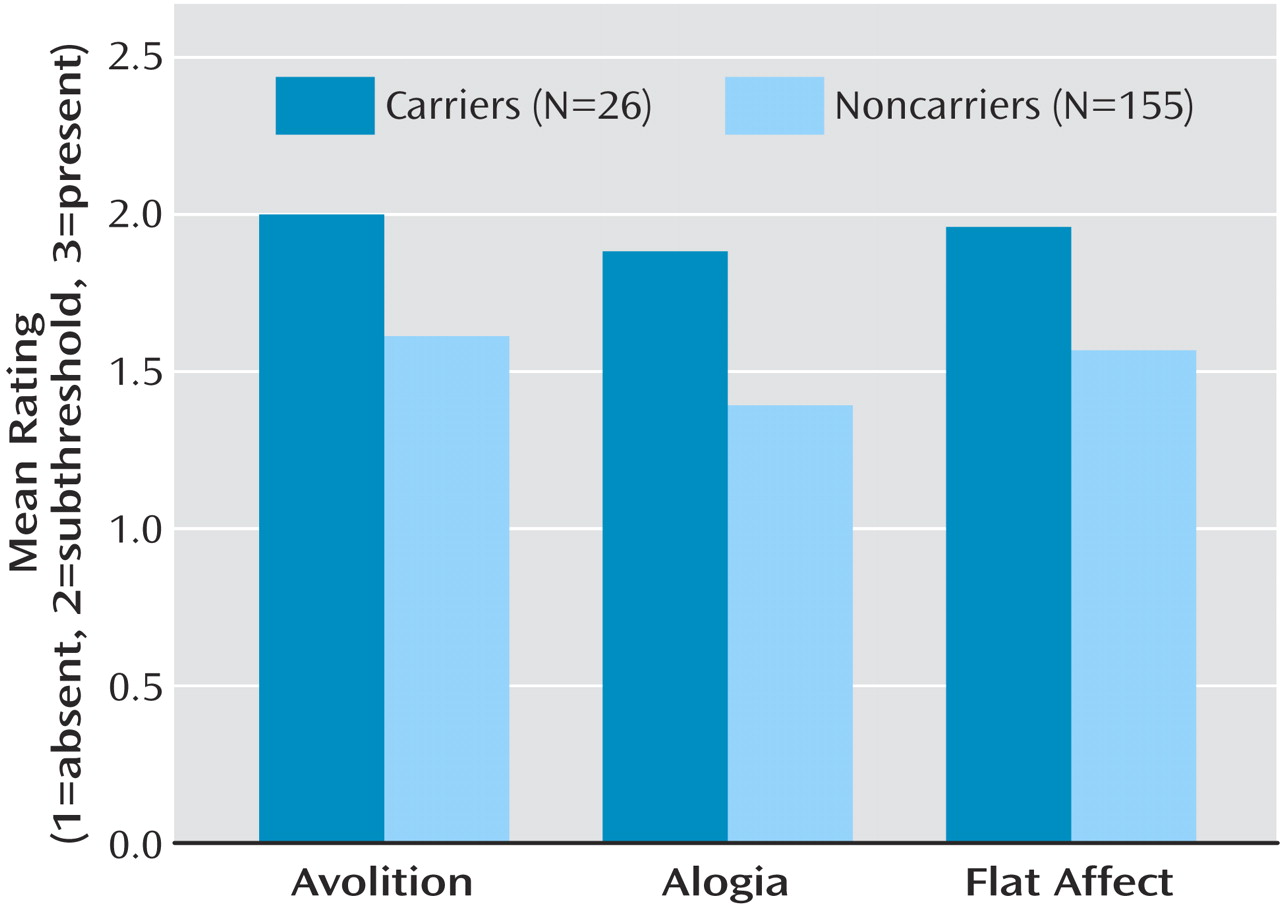
The MANOVA revealed significant group differences (F=3.84, df=3, 177, p=0.01), and post hoc t tests revealed that carriers had significantly higher ratings than noncarriers on all three items (
Figure 1): avolition (t=2.08, df=180, p<0.04), alogia (t=2.60, df=180, p<0.02), and flattened affect (t=2.45, df=180, p<0.02). Ratings for all three items were combined to produce an overall negative symptom rating. A univariate ANOVA comparing the mean overall negative symptom rating of carriers (mean=5.85, SD=2.09) with that of noncarriers (mean=4.57, SD=1.77) indicated that CTCTAC carriers had higher overall ratings (F=10.93, df=1, 180, p=0.001). This difference remained significant after covarying for the global neurocognitive measure and the WRAT-3 (F=5.02, df=1, 131, p<0.03).
Discussion
We detected a significant association between the CTCTAC haplotype and lifetime severity of negative symptoms in patients with schizophrenia. The significant results of the ANCOVA indicate that this relation cannot be accounted for entirely by the association between dysbindin and cognition that we previously identified
(6). Further, the association between dysbindin and cognition has also been detected in a group of healthy volunteers, who would not be expected to exhibit negative symptoms. Moreover, a comparison of other symptom clusters from the SCID (positive, depressive, and manic symptoms) revealed no differences between carriers and noncarriers (data not shown), suggesting that this haplotype is not associated with global symptom severity but with negative symptom severity.
Recent data indicating a negative correlation between neuronal dystrobrevin and the glutamate transporter in schizophrenia
(7) suggest that the dysbindin genotype influences glutamatergic function. Since glutamatergic antagonists such as ketamine can produce negative symptoms in healthy individuals and exacerbate negative symptoms in schizophrenia
(8), the dysbindin genotype may exert its effects on negative symptoms through a glutamate-receptor-related mechanism. Further studies elucidating the relation between the glutamatergic system and dysbindin genotype are warranted.
Several limitations of the current study should be noted. First, although the SCID assesses lifetime history of negative symptoms, other tools, such as the Scale for the Assessment of Negative Symptoms or the Schedule for the Deficit Syndrome, are more comprehensive. These assessments were not available in the present data set. Second, we analyzed only a single dysbindin risk haplotype in our study. Although several other DTNBP1 haplotypes have been associated with schizophrenia, the CTCTAC haplotype is the only haplotype demonstrated to influence neurocognition, and it is the only haplotype associated with schizophrenia in our patients. It should be noted, however, that there are no data on the biological significance of this haplotype from in vitro assays. Therefore, other haplotypes, or functional alleles if detected, should be tested for association with clinical symptoms in independent groups of subjects.
In summary, we have detected a significant association between a dysbindin risk haplotype and severity of negative symptoms in a relatively large group of patients with schizophrenia or schizoaffective disorder. These data suggest that the dysbindin genotype may modify the clinical presentation of schizophrenia and may represent an initial step toward a molecular classification of illness.