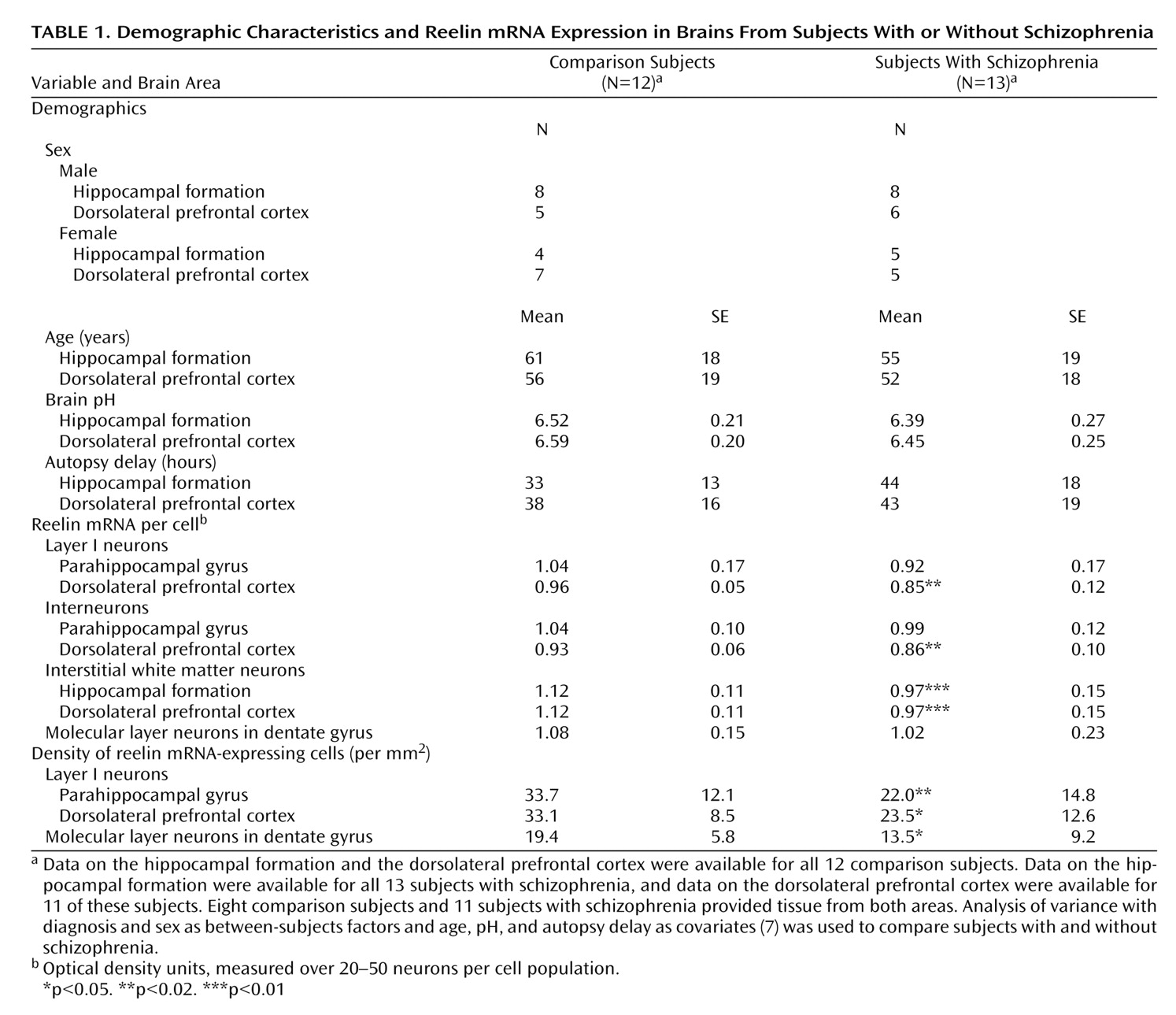
Our data confirm that reelin expression is reduced in schizophrenia
(2–
5,
7) and show its cellular origin in the hippocampal formation and the dorsolateral prefrontal cortex. The distribution in the dorsolateral prefrontal cortex is similar to that in the superior temporal gyrus
(7). The reduction in schizophrenia is apparent in terms of both reelin mRNA per cell and density of reelin-positive cells (
Table 1). With regard to the latter finding, other studies show that there is no loss of the neurons themselves in schizophrenia
(9,
10). Thus, the present results are indicative of a down-regulation of reelin expression.
In both areas studied, interstitial white matter neurons expressed less reelin mRNA, drawing renewed attention to this developmentally critical cell population—the presumed remnants of the cortical subplate—previously implicated in schizophrenia (see reference
7). In the dorsolateral prefrontal cortex, reelin mRNA was also reduced in layer I neurons, the survivors of Cajal-Retzius cells, essential for neuronal migration and lamination
(1). Possibly, reduced reelin might be causal to a developmental anomaly affecting interstitial white matter neurons and Cajal-Retzius cells, but, equally, it might be consequential or unrelated to it. Regardless, since both neuron populations form synaptic contacts and persist in adulthood, and given that reelin has key roles in plasticity, the abnormality likely has ongoing effects on the functioning of neural circuits. Note that although reelin mRNA is not detected in pyramidal neurons, reelin is
(11), perhaps because the neurons internalize the protein
(12) after its secretion by the GABA-ergic cells with which they are synaptically connected. If so, reelin may have a role linking GABA-ergic with glutamatergic aspects of the disease pathophysiology, including its synaptic component
(6). The correlations between reelin load and the presynaptic protein transcripts provide some empirical support for this proposal.
Although layer I neurons and most cortical interneurons are GABA-ergic, most interstitial white matter neurons are not, at least in rodents
(13). Moreover, the various reelin-expressing cell populations differ in place and time of origin
(14). Thus, our data suggest that the involvement of reelin in schizophrenia is not related directly to transmitter phenotype or embryological history of the neurons concerned. Instead, it may reflect an intrinsic property of the gene and its regulation. Possibilities include epigenetic factors
(5) or an interaction with susceptibility genes, which themselves have effects on synaptic plasticity
(15).