The antisaccade task requires the initiation of a rapid eye movement away from a peripheral visual stimulus. Patients with schizophrenia show a higher frequency of reflexive errors than do healthy subjects, as well as longer latency and less spatial accuracy for correct antisaccades
(1–
3). Antisaccade performance has been linked to frontal-striatal functioning in healthy individuals and dysfunction in schizophrenia
(2,
3). Schizophrenic patients’ first-degree relatives share some of these deficits
(1,
4–6), and more than 50% of the variance in antisaccade errors is due to additive genes
(7), suggesting that this task taps genetic vulnerability for schizophrenia.
Monozygotic twins discordant for schizophrenia are an important population in which to test the validity of a putative endophenotype. This population allows one to address whether a candidate endophenotype is associated with the genetic predisposition for the illness, its expression, or both.
To our knowledge, no study of antisaccade performance in monozygotic twins discordant for schizophrenia has been published. We, therefore, aimed to investigate whether antisaccade deficits are observed in this population. We included an oculomotor control condition, the prosaccade task, on which no between-group differences were expected.
Method
Ethical approval and written informed consent were obtained. The study group comprised 10 Caucasian monozygotic discordant twin pairs and 10 Caucasian monozygotic comparison twin pairs (six male and four female pairs in each group). The exclusion criteria were history of neurological illness, head injury with loss of consciousness for more than 1 minute, and substance misuse or dependence in the last year. One 29-year-old schizophrenic patient (but not her co-twin) was excluded because of strabismus. Zygosity was determined by using a twin likeness questionnaire and assessment of 12 independent and highly polymorphic microsatellite markers. In each of the discordant pairs, one twin (the proband) met the DSM-IV criteria for schizophrenia while the co-twin (the nonschizophrenic co-twin) was free of any psychotic disorder. The probands’ symptoms were rated by using the Scale for the Assessment of Positive Symptoms (SAPS)
(8) and the Scale for the Assessment of Negative Symptoms (SANS)
(9) (intra- and interrater reliability: intraclass correlation coefficient=0.91–0.93). On average, 9.93 years (SD=8.11) had elapsed since the onset of the probands’ illness. The comparison twins were without lifetime DSM-IV axis I diagnoses and were free of family history, to their second-degree relatives, of psychosis
(10).
The saccade tasks were identical to those in a previous protocol
(11). Testing took place either in the laboratory or at the participants’ homes (five discordant and three comparison pairs). For all pairs, both members were seen in the same environment (laboratory or home). A trial consisted of the target in the center of a computer monitor for a random duration of 1,000–2,000 msec and, subsequently, in one of four peripheral locations (±6°, ±12°) for 1,000 msec. Each task comprised 60 trials. For prosaccades, the participants were instructed to follow the target as accurately as possible. During the antisaccade task, the participants were instructed that while the target was in the center of the screen they should look at the target and that when the target jumped to the side they should look at the location exactly opposite the target, as though at its mirror image. Eye movements were recorded by using infrared oculography sampled at 500 Hz. The data were scored by a rater (U.E.) blind to group status (inter- and intrarater reliability: r=0.91–0.99). The antisaccade data of one male discordant pair could not be used because of poor quality. Saccades were detected by using minimum amplitude (1°), velocity (30°/second), and latency (100 msec) criteria. We calculated latency (in milliseconds), gain (saccade amplitude divided by target amplitude, expressed as a percentage), and for antisaccades, error rate (reflexive saccades divided by total number of valid trials, expressed as a percentage).
Group differences were analyzed by using a regression model. Inferences were based on standard errors that were robust against correlations within twin clusters, variance heterogeneity, and departures from normality
(12) (Stata Corp., College Station, Tex.). The model uses group as the independent variable and the saccade measures as the dependent variables. The alpha level for overall group comparisons was 5%. Significant differences were followed by three Bonferroni-adjusted post hoc comparisons (alpha level, 0.05/3=0.017). Gender and age were included as covariates. Effect sizes were defined as the estimated group difference divided by the square root of the average of the variances within the proband and the unaffected co-twin groups. Pearson correlations between the SAPS/SANS scores and the saccade variables were calculated.
Results
The probands’ treatment included clozapine (N=4), olanzapine (N=2), risperidone (N=1), and none (N=2). Their mean SAPS and SANS scores were 5.22 (SD=3.38) and 10.11 (SD=6.79), respectively. Of the nonschizophrenic co-twins, seven had past psychiatric histories: major depressive disorder (N=3), major depression and obsessive-compulsive disorder (N=1), depression and schizotypal personality disorder (N=1), and schizotypal personality disorder (N=2). None had active axis I diagnoses at assessment or was taking medication.
The twin groups did not differ in age (discordant: mean=31.20, SD=9.90; comparison: mean=33.90, SD=12.12) or parental socioeconomic status according to the U.K. National Statistics Socio-Economic Classification (http://www.statistics.gov.uk/methods_quality/ns_sec) (discordant: mean=2.50, SD=0.71; comparison: mean=2.38, SD=0.72) (p>0.36, two-tailed t test).
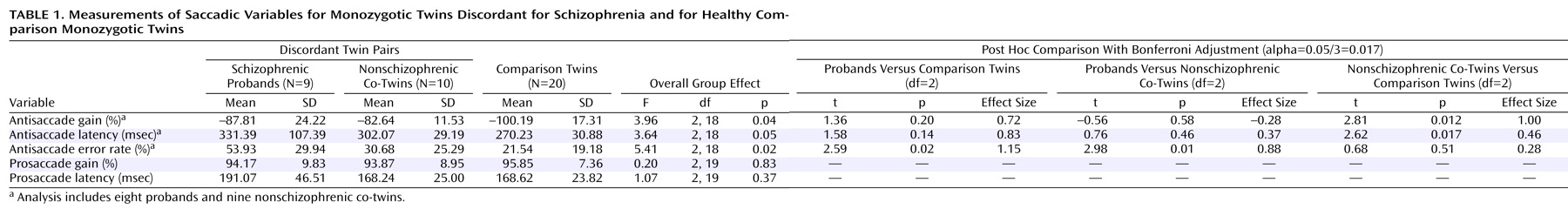
For the saccadic variables, the normality and homogeneity of variance were assessed by using box plots. While normality appeared a reasonable assumption, variability in antisaccade latency was somewhat higher among the probands, although there were no outliers (defined as having a score more than 1.5 interquartile ranges outside either quartile). The 25th, 50th, and 75th percentiles of antisaccade latency were 270.61, 279.09, and 410.77 msec for the probands; 279.30, 300.47, and 317.72 msec for the co-twins; and 249.76, 270.82, and 302.00 for the comparison subjects.
Significant group effects were obtained for antisaccade gain, latency, and error rate (
Table 1). On the antisaccade error rate, the probands differed at a nearly significant level from the healthy comparison twins and significantly from the co-twins. The nonschizophrenic co-twins displayed significantly less (i.e., hypometric) gain and greater latency than the comparison twins. There were no significant group differences on prosaccade performance.
The SANS score was correlated with antisaccade errors (r=0.72, N=8, p=0.05), and the SAPS score was correlated with prosaccade gain (r=–0.74, N=9, p=0.03) (for other correlations, p>0.10).
Discussion
The nonschizophrenic co-twins of schizophrenic patients displayed significant impairments in antisaccade gain and latency. They made antisaccades of hypometric amplitudes (with a large effect size) and had longer latencies (with a medium effect size) relative to the comparison twins, but they did not differ from the probands on these variables. Similar deficits have been demonstrated in schizophrenic patients and their first-degree relatives
(1,
4,
5), suggesting that these deficits may serve as schizophrenia endophenotypes in genetic studies. These differences are particularly striking given that all groups displayed comparable prosaccade gain and latency.
The probands’ antisaccade gain and latency deficits (with medium-to-large effect sizes) failed to reach statistical significance, possibly because of the probands’ smaller group and large variance. In the proband group, the error rates were correlated with negative symptom levels
(2), and prosaccade gain was correlated with positive symptom levels
(11).
The limitations of this study include the fact that we did not study monozygotic twins concordant for schizophrenia or singletons. This might be relevant, given the suggestion that environment may have a particularly strong role in etiology for monozygotic discordant pairs
(13). Additionally, the study group was small, because monozygotic discordant twins are rare. Finally, the group differences may have been confounded by the nonschizophrenic co-twins’ past psychiatric diagnoses.