Method
Study Overview
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was a study funded by the National Institute of Mental Health to evaluate the course and outcome of patients with bipolar disorder
(5) . The study combined naturalistic and randomized approaches to assess somatic and psychosocial interventions in the treatment of all phases of bipolar illness. Regardless of whether patients were participating in randomized trials, they received the same ongoing assessments of treatment and outcome. Our current study examined outcomes of patients involved in the “standard care pathway,” in which patients were followed naturalistically and treated by using “best practices” guidelines
(5) .
Participants
Patients had to meet the DSM-IV criteria for bipolar I or II disorder, bipolar disorder not otherwise specified, or cyclothymia. Patients were required to be at least 15 years of age. All participants provided written informed consent. Patients entered the study in syndromal, subsyndromal, or recovered states. A more complete description of the STEP-BD participants was reported by Kogan et al.
(7) .
Intake Assessments
The Affective Disorder Evaluation
(8) is an intake assessment tool intended for routine use by practicing clinicians. It includes numerous questions on present illness, past psychiatric history, family history, medical history, and mental status. In addition, it includes a modified version of the mood and psychosis modules from the Structured Clinical Interview for DSM-IV (SCID). For the current study, the Affective Disorder Evaluation was used to assess age at study entry, age at onset of mood episodes, number of prior episodes, periods of recovery, current medications, and current mood state. A study psychiatrist completed the Affective Disorder Evaluation with the patient at study entry.
The Mini International Neuropsychiatric Interview (MINI) is a semistructured interview designed to identify major axis I psychiatric disorders. In validation and reliability studies, the MINI was compared to the patient edition of the SCID for DSM-III-R and found to have acceptably high validity and reliability scores
(9) . In the present study, the MINI Plus Version 5.0, administered to the patient by study research personnel (psychologists, social workers, or psychiatric nurses), was used to confirm the bipolar diagnosis. Diagnostic discrepancies between the Affective Disorder Evaluation and MINI were resolved by consensus discussion between the research staff member and the study psychiatrist.
Follow-Up Assessments
The Clinical Monitoring Form
(10) is a 1-page assessment tool used by study clinicians to document symptom and treatment characteristics of patients at each clinical visit. It consists of modified versions of the SCID current mood modules, associated symptoms, current medications, compliance and adverse effects, laboratory data, and summary scores (i.e., clinical status, Clinical Global Impression severity rating, and Global Assessment of Functioning Scale [GAF] score). Intraclass correlations of physicians’ interrater reliability with gold standard ratings for the depression and mania items of the Clinical Monitoring Form ranged from 0.83 to 0.99. The treating psychiatrists were periodically monitored to ensure that the standards for ratings with the Clinical Monitoring Form were maintained
(5) .
The number of Clinical Monitoring Forms generated over the course of treatment varied among individual patients, as treatment was naturalistic and patients were seen as often as the treating psychiatrist deemed clinically appropriate. STEP-BD clinicians assigned one of eight clinical states to each patient at each clinic visit. Four of the clinical status definitions corresponded to DSM-IV criteria for major depression, mania, hypomania, and mixed episodes. The remaining four clinical definitions characterized subsyndromal or recovered states and were assigned according to the number and duration of mood symptoms present (if any).
In addition to the preceding measures, researchers administered the Young Mania Rating Scale
(11) and Montgomery-Åsberg Depression Rating Scale (MADRS)
(12) to patients at study entry to determine the severity of illness. Scores on these instruments were used as predictors of rapid cycling during the prospective study year.
Cycling Determination at Study Entry
Data from the Affective Disorder Evaluation were used to determine the number of prior affective episodes. Patients were described as having prior rapid cycling if they had experienced four or more mood episodes in the prior 12 months, with depressive episodes lasting at least 2 weeks, manic episodes lasting at least 1 week, or hypomanic episodes lasting at least 4 days. Patients who did not meet these criteria were designated as nonrapid cyclers. The groups with and without rapid cycling were further stratified by bipolar I versus bipolar II diagnosis.
Treatment
The pharmacotherapy procedures were based on expert consensus guidelines, such as those published by APA
(13) and others
(14) . The treatment choices were presented in tiers, corresponding to treatments considered “first line” in one or more published guidelines, “second line,” etc. Physicians and patients consulted a “menu of reasonable choices” in deciding on augmentations or substitutions; for more information, see reference 15.
Cycling Determination and Outcome Groups During Prospective Year
Because we considered that any mood changes were clinically important, not just recurrences at rates greater than four episodes per year, we created four outcome groups based on patients’ prospectively observed cycle frequencies. Patients were categorized as having no episodes, one episode, two or three episodes, or rapid cycling (four or more episodes). Patients who did not complete 12 months of treatment were placed in a fifth category, “dropped“ (terminated the study early), so that we could compare the clinical characteristics of patients who were and were not able to complete 12 months of treatment.
Phase changes were determined by using the Clinical Monitoring Form. Episodes were considered separate if a patient’s mood changed from one state to one of opposite polarity, or if the patient had achieved euthymia for at least 8 weeks and then experienced a mood episode meeting the DSM-IV criteria for mania, hypomania, a mixed state, or depression. Moods that changed in intensity or quality within the same polarity (e.g., from hypomania to mania or from mania to a mixed state) were not counted as phase changes.
Statistical Analyses
The four outcome groups (no episodes, one episode, two or three episodes, rapid cycling) were first compared on gender, bipolar subtype, age, age at onset of illness, and prior rapid cycling status by means of t tests and chi-square analyses. In addition, entry scores on the MADRS, Young Mania Rating Scale, and GAF were used as predictors of cycling outcome. Using survival analytic and logistic regression models, we examined whether prior rapid cycling status or clinical and demographic variables (history of rapid cycling, age at onset, entry MADRS, Young Mania Rating Scale, and GAF scores, age at study entry, gender, and bipolar subtype) predicted the likelihood of 1) dropping out during the study year or 2) having no episodes, one episode, two or three episodes, or four or more episodes. To identify naturally occurring cutoff points that might separate cycling categories, we plotted the number of prospectively occurring mood episodes against baseline scores on the MADRS, Young Mania Rating Scale, and GAF and age at illness onset.
Depressed patients would be expected to be seen by their psychiatrists more often, and in turn, psychiatrists would have a greater opportunity to observe cycling. Therefore, we examined the relation of baseline symptom scores to prospectively observed cycling with the number of psychiatric visits covaried.
To determine the relationship between cycling outcome and antidepressant use, we entered antidepressant use and baseline MADRS score into a logistic regression model to predict membership in one of the four prospective outcome groups. A single multivariate logistic regression model determined which predictors were redundant. All statistical tests were two-tailed.
Results
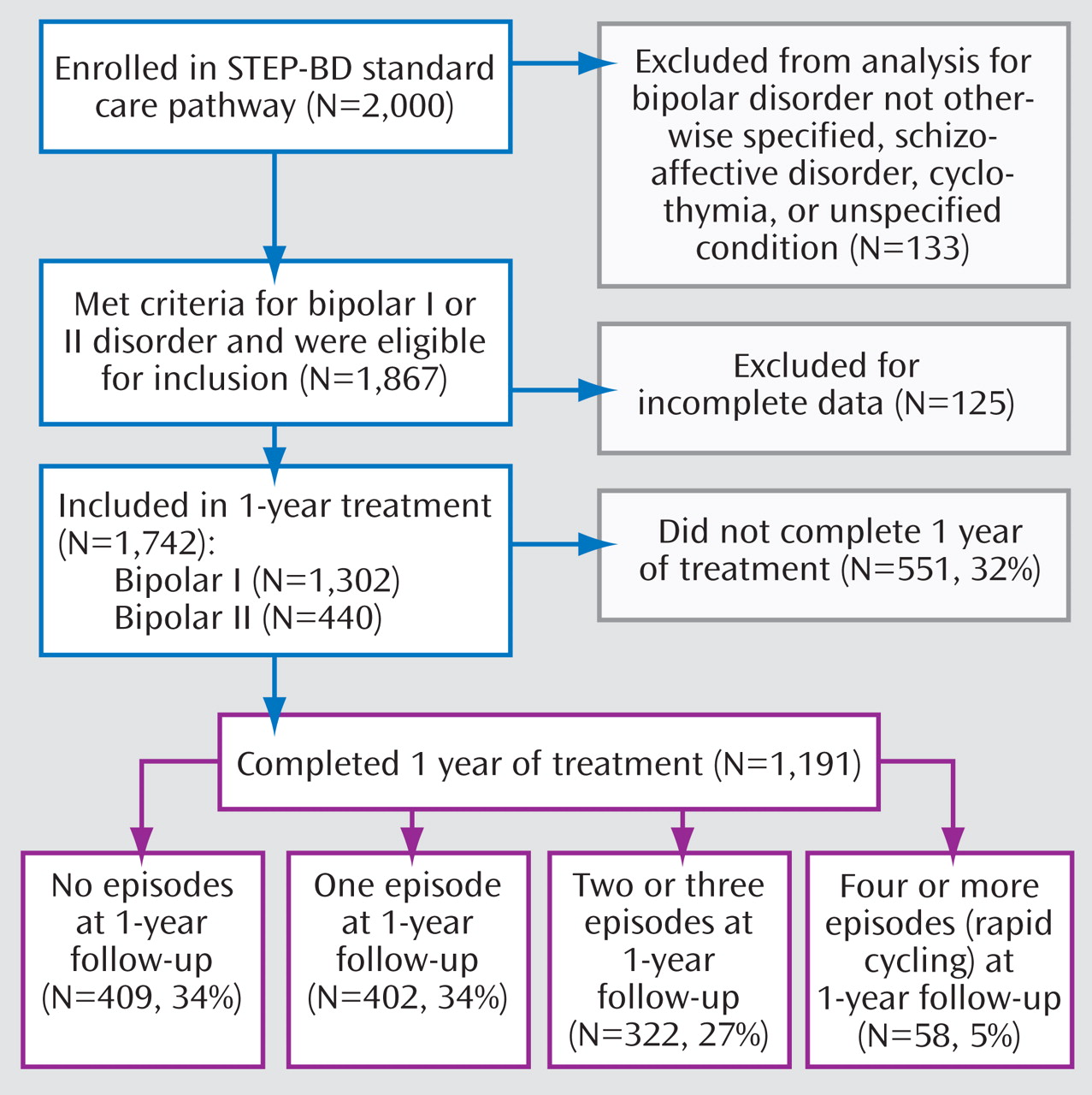
Figure 1 presents an overview of the study. The study group consisted of the first 2,000 patients enrolled in STEP-BD and assessed between November 1999 and April 2002. Patients diagnosed with bipolar disorder not otherwise specified, schizoaffective disorder, cyclothymia, or unspecified condition were excluded from our analysis, as were patients with incomplete data. Of the 1,742 patients included, 75% were diagnosed with bipolar I disorder and 25% were diagnosed with bipolar II disorder. A total of 551 patients (32% of the original 1,742) did not complete 1 year of treatment and were designated as dropped. During the 1 year of prospective follow-up, only 58 (5%) of the remaining 1,191 patients had four or more mood disorder episodes (DSM-IV rapid cycling) (
Figure 1 ).
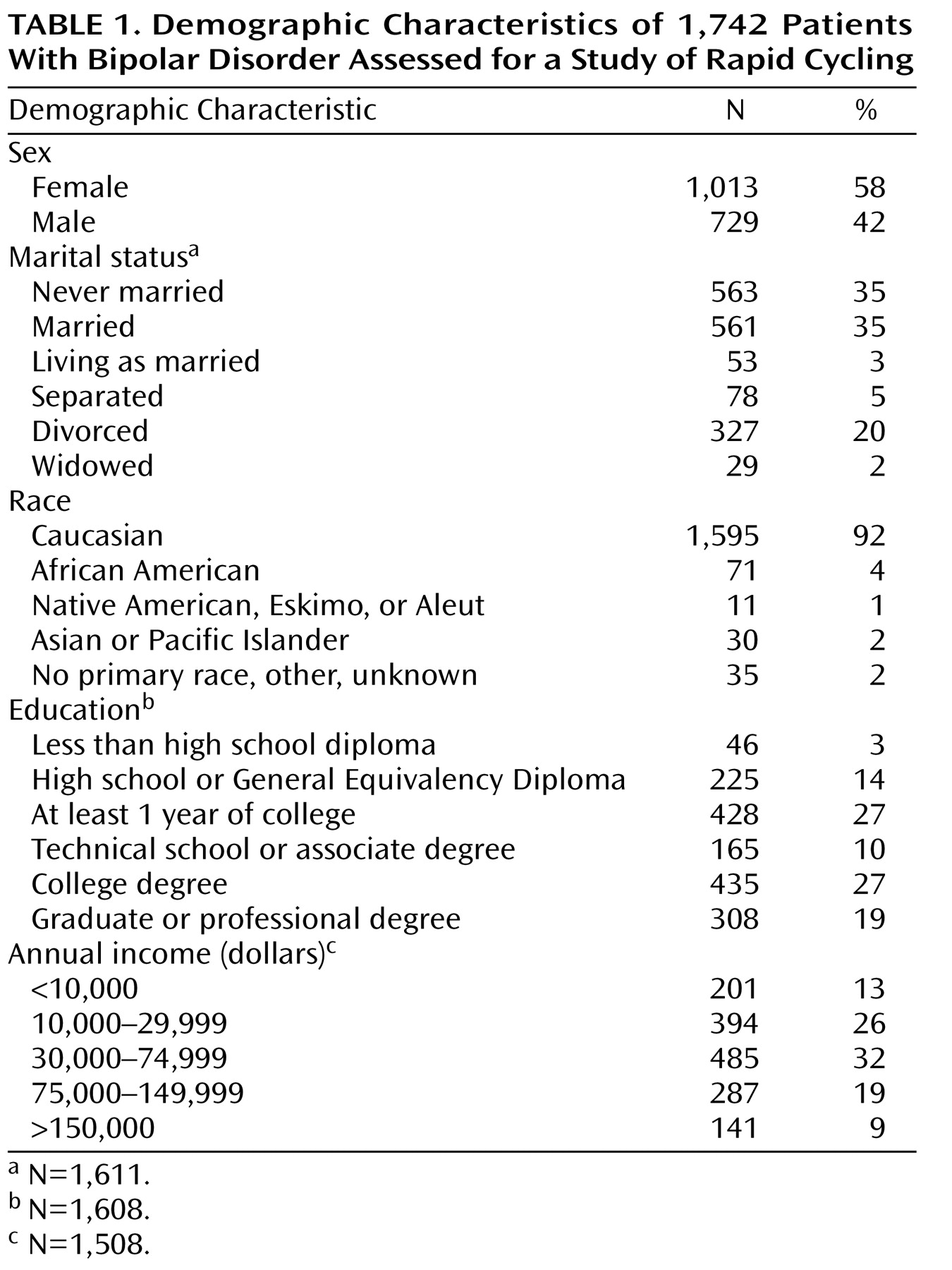
The mean age of the patients in the study was 40.4 years (SD=12.9). Other demographic characteristics are presented in
Table 1 . The majority of the study group were Caucasian, female, and college educated.
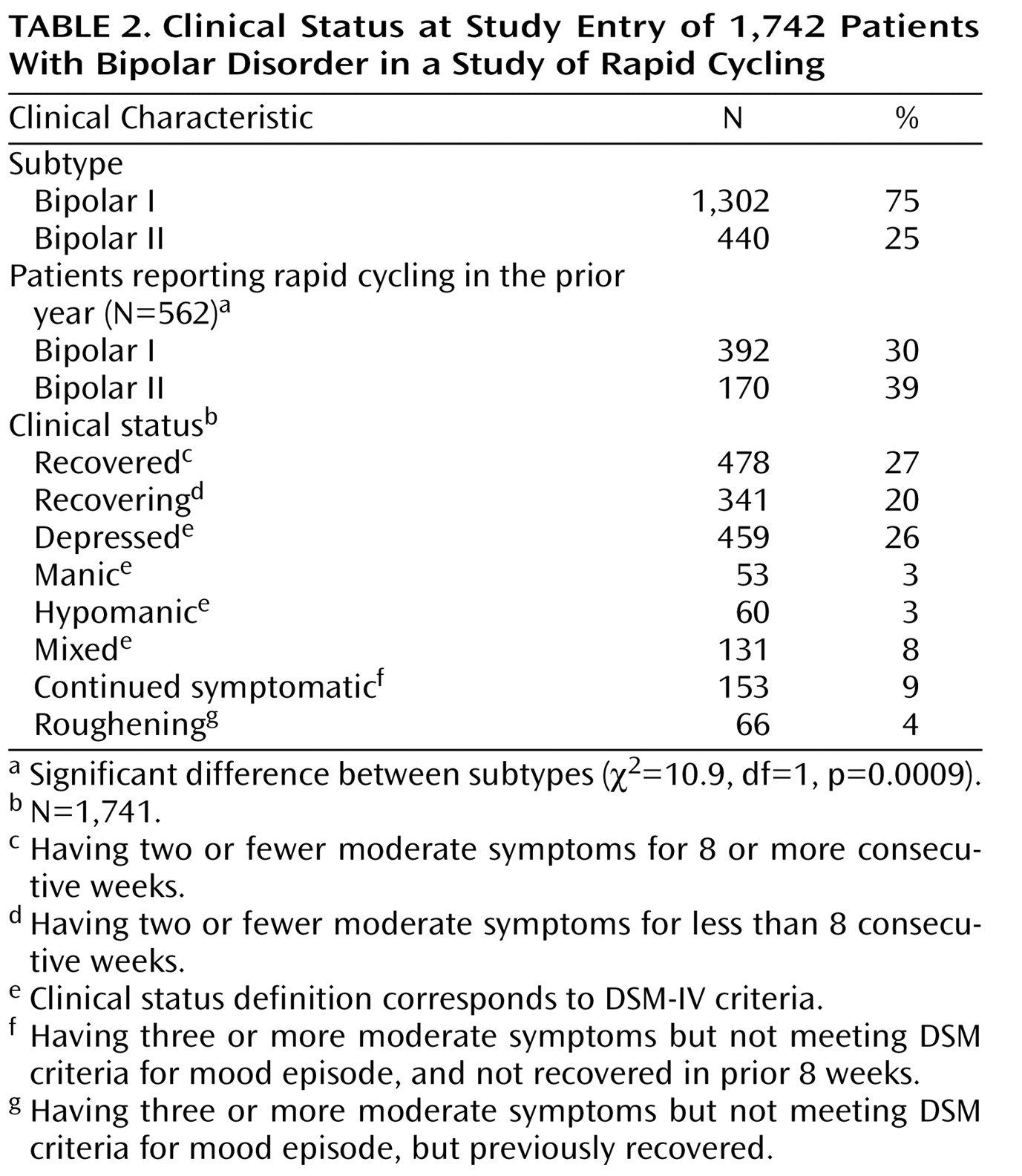
Table 2 presents clinical features of the participants at study entry. Patients entered the STEP-BD in a variety of symptomatic states, with approximately one-half of the group classified as either recovering or recovered. A total of 562 of the original 1,742 patients (32%) reported rapid cycling in the 12 months before study entry. The proportion of patients who reported rapid cycling in the prestudy year was higher for patients with bipolar II disorder than for those with bipolar I disorder (
Table 2 ).
Analysis of Dropouts
Multivariate logistic regression revealed that age at study entry (χ 2 =5.6, df=1, p=0.02), GAF score at study entry (χ 2 =5.6, df=1, p=0.02), and prior history of rapid cycling (χ 2 =6.5, df=1, p=0.01) were independent predictors of dropping out of the study, while age at onset of illness, entry scores on the MADRS and Young Mania Rating Scale, gender, and bipolar subtype were not. Kaplan-Meier survival analysis revealed that patients with a history of prior rapid cycling were slightly more likely to drop out—and to drop out earlier—than patients without prior rapid cycling. Among the 562 patients with prior rapid cycling, 206 (37%) did not complete 1 year of treatment, while 343 (29%) of the 1,180 patients without a history of rapid cycling did not complete 1 year (χ 2 =8.7, df=1, p=0.003).
Predictors of Cycling During 1-Year Prospective Period
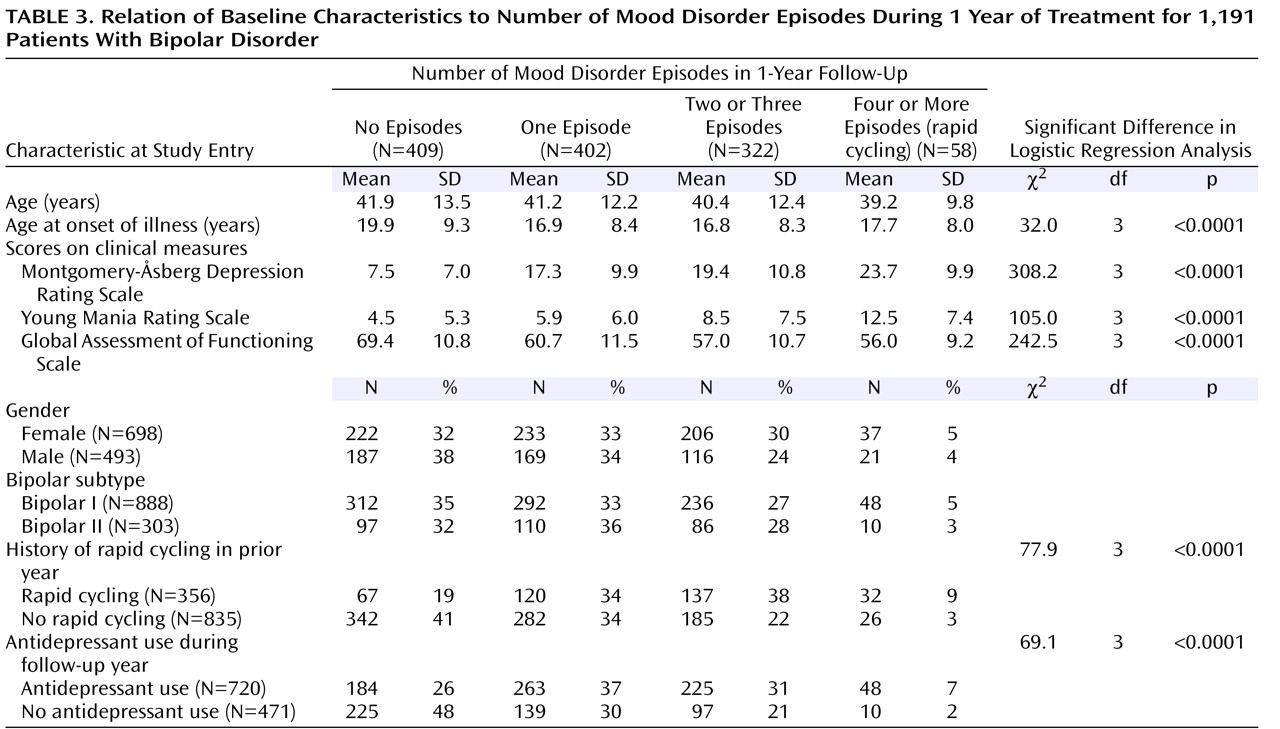
Demographic and illness variables
Table 3 shows demographic and clinical risk factors for episode recurrences and their relationships to cycling outcome. Younger age at onset of bipolar illness was a significant predictor of increased recurrence. Patients who had no prospectively observed mood episodes were older at the onset of illness, while patients in all of the other cycling groups developed bipolar illness significantly earlier. Bipolar subtype was not predictive of cycling outcome. Female gender also did not emerge as a predictor of the development of frequent cycling (two or three episodes) or rapid cycling (χ
2 =7.7, df=3, p=0.053).
Prior rapid cycling
Compared to patients who had not been classified as rapid cyclers during the prior year, patients who entered the study with a recent history of rapid cycling were more likely to have at least one prospectively observed episode during the follow-up year. Of the 356 patients with rapid cycling in the prestudy year, only 67 (19%) maintained stability throughout the 12-month study, compared to 342 (41%) of the patients without rapid cycling in the prestudy year (
Table 3 ).
Baseline clinical severity
Higher entry MADRS scores (covering the week before study entry) predicted a greater likelihood of experiencing one episode, two or three episodes, or rapid cycling in the follow-up year (
Table 3 ). Likewise, patients with higher entry scores on the Young Mania Rating Scale were more likely to have frequent or rapid cycles in the follow-up year. In an analogous fashion, lower GAF scores at entry predicted a greater likelihood of experiencing frequent or rapid cycling.
Multivariate prediction of rapid cycling
Consistent with our univariate analyses, multivariate logistic regression revealed that prior rapid cycling as well as intake scores on the MADRS, Young Mania Rating Scale, and GAF were independent predictors of rapid cycling, versus stable status, in the prospective year. Odds ratios and 95% confidence intervals (CIs) showed significant effects for prior rapid cycling (odds ratio=4.4, 95% CI=2.1–9.0, p<0.0001), intake MADRS score (odds ratio=1.15, 95% CI=1.1–1.2, p<0.0001), intake score on the Young Mania Rating Scale (odds ratio=1.1, 95% CI=1.0–1.1, p=0.0007), and intake GAF score (odds ratio=0.95, 95% CI=0.92–0.98, p=0.002). Age, age at onset, and bipolar subtype were not associated with future rapid cycling once the baseline clinical variables were covaried. An interesting finding was that the same predictors significantly distinguished between patients who had two or three episodes and those with no prospective episodes.
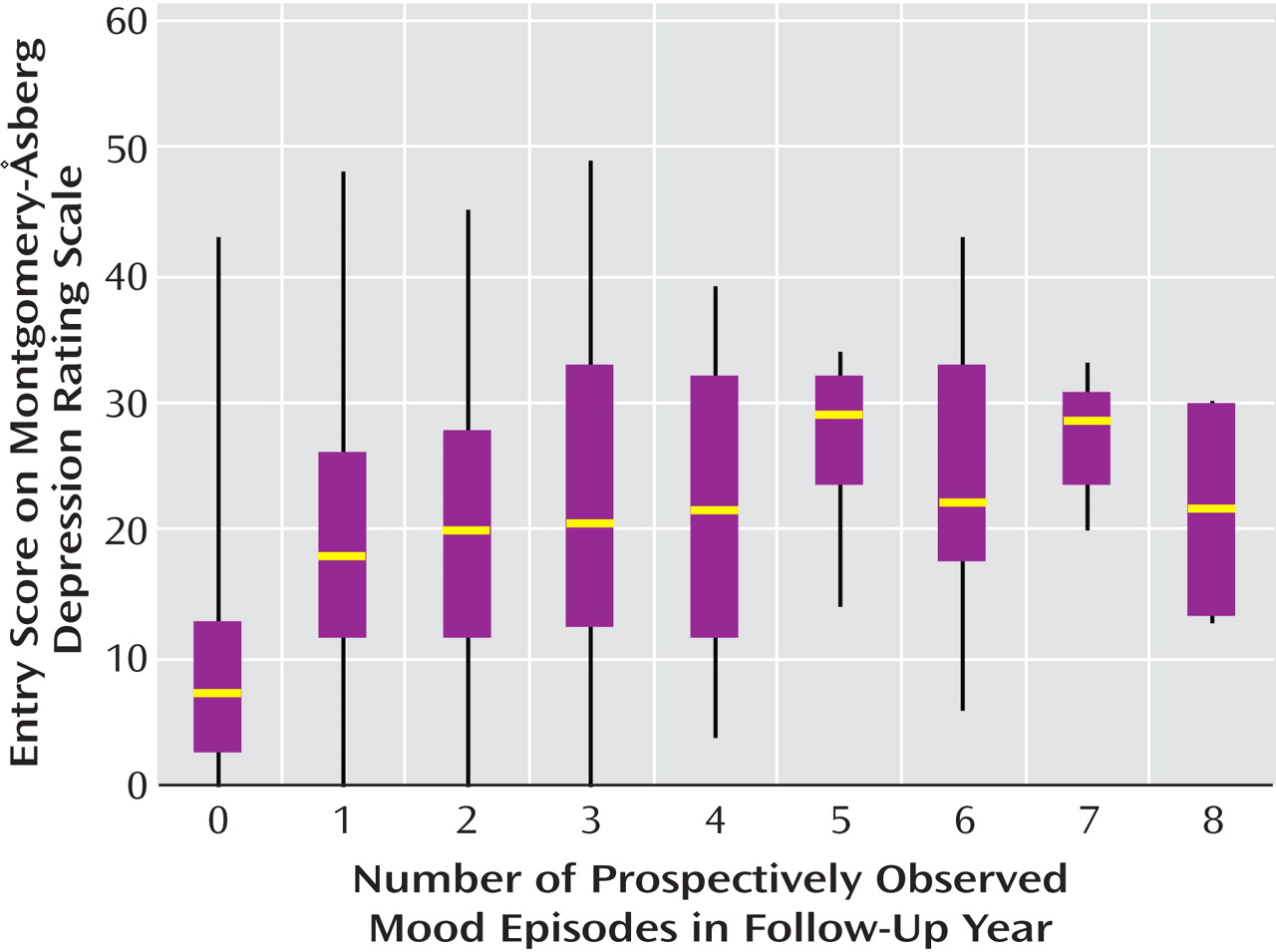
The association between baseline illness severity and number of prospectively observed episodes appeared to be linear. It did not appear that patients with four or more episodes were uniquely distinguishable from patients with one to three episodes in terms of intake scores on the MADRS (
Figure 2 ), Young Mania Rating Scale, or GAF or in terms of age at illness onset.
Number of psychiatry visits
As expected, the number of psychiatry visits varied significantly by prospective cycling status. Stable patients had the fewest visits (mean=7.0, SD=3.8) while rapid cyclers had the most (mean=16.3, SD=6.3). Patients with high initial MADRS scores were seen more often during follow-up (F=45.8, df=1, 1592, p<0.0001), but Clinical Monitoring Form visits (χ 2 =155.6, df=3, p<0.0001) and MADRS scores (χ 2 =178.2, df=3, p<0.0001) independently predicted cycling status at follow-up. Entry scores on the Young Mania Rating Scale were unrelated to the number of visits (F=73.5, df=1, 1585, p=0.02), but Young scale scores (χ 2 =85.7, df=3, p<0.0001) and visits (χ 2 =200.4, df=3, p<0.0001) were independently predictive of cycling status after visit count was covaried.
Effects of Antidepressant Use
Antidepressant use was common during the first year of treatment. A total of 720 patients (60%) received antidepressants at some time during the follow-up year, and 471 (40%) did not. Of the patients who took antidepressants, 45% took them with a mood stabilizer (lithium, divalproex, lamotrigine, or carbamazepine), 6% took them with an atypical antipsychotic (risperidone, olanzapine, quetiapine, ziprasidone, or clozapine), 43% took them with both a mood stabilizer and an atypical antipsychotic, and 6% took an antidepressant only.
Antidepressant use during the follow-up year was consistently associated with more frequent cycling (
Table 3 ). After controlling for MADRS score at study entry, we found that patients who received antidepressants were 3.8 times (95% CI=1.7–8.5, p=0.001) as likely to experience rapid cycling, 2.0 times (95% CI=1.4–2.9) as likely to have two or three episodes (p=0.0001), and 1.7 times (95% CI=1.2–2.3) as likely to have one episode (p=0.001) during the follow-up year compared to patients who did not receive antidepressants.
Discussion
The present study examined the course of cycling in a large group of bipolar patients (N=1,742) over the course of 1 year of treatment. Although we found the occurrence of four or more episodes in the follow-up year to be rare (only 5% of patients), recurrences at lower but still clinically relevant rates continued: 61% of the participants experienced between one and three episodes over the 12 months of follow-up.
The observed rate of DSM-IV-defined rapid cycling was much lower in the follow-up year (5% of patients) than in the year before entry into STEP-BD (32%). This difference is similar to the findings of Coryell et al.
(15,
16), who observed that the percentage of rapid-cycling patients decreased from approximately 19% in the first year of treatment to 5% over the next 2–5 years. In the second study by Coryell et al.
(16), in which patients were followed for approximately 15 years, four of five cases of rapid cycling ended within 2 years of their onset. Collectively, these results support the notion that rapid cycling is a transitory phenomenon in the course of bipolar illness.
The low prevalence of rapid cycling in our prospective study may have been attributable to several other factors. First, rapid cycling determined at study entry was diagnosed retrospectively, while the prospective observation used strict DSM-IV mood criteria and a minimum duration of 8 consecutive weeks of euthymia between episodes. Second, patients’ recollections of the number of cycles they experienced in the prior year may have been subject to retrospective bias in reporting (i.e., exaggeration of the number of episodes). Third, the use of a week-to-week prospective assessment method, such as the LIFE
(17), may have revealed episodes that were not captured by psychiatric visits given as clinically needed. Last, the “model practice procedures” treatment employed by STEP-BD psychiatrists specially trained in treating mood disorders may have had a positive impact on the outcome of patients with a history of rapid cycling.
Nevertheless, our findings demonstrate that a reported history of recent rapid cycling is prognostically significant. Compared to patients without rapid cycling in the year before the study, patients who reported rapid cycling in the prior year were far less likely to be stable during the course of the follow-up year (41% versus 19%, respectively) and were more likely to have prospectively observed cycling, regardless of cycling frequency. Whereas patients with prior rapid cycling and patients without prior rapid cycling were equally likely to have one episode in the follow-up year (34% for each group), prior rapid cyclers were more likely to experience two or three episodes (38% versus 22%) and three times as likely to experience rapid cycling during follow-up (9% versus 3%). In our multivariate regression analysis, a history of rapid cycling emerged as the strongest predictor of cycling in the prospective year, regardless of cycling frequency.
The predictive validity of recent cycling is consistent with results reported by Bauer et al.
(3), whose rapid-cycling patients had significantly more episodes than non-rapid-cycling patients during 12 or more months of prospective follow-up. From a clinical standpoint, we would not anticipate differences in the management of patients experiencing two or three episodes in a year and those with rapid cycling. Our data suggest that patients who report a recent history of rapid cycling are more vulnerable to cycling in the immediate future and that caution should be exercised in prescribing any medication with potential cycle-promoting activity.
Severity of illness at study entry proved to be a significant predictor of cycling outcome. Patients entering the study more depressed, more manic, or with poorer functioning were more likely to cycle in the follow-up year. The differences observed between the scores of stable patients and those with cycling episodes were clinically meaningful: entry MADRS scores for patients who had any prospectively observed episodes were in the moderately depressed range, whereas those who remained stable for the prospective year had scores indicating euthymia. On the Young Mania Rating Scale, clinically significant elevations in scores portended later rapid cycling, i.e., entry scores indicating mild hypomania were found in patients who later became rapid cyclers. Finally, the GAF scores revealed a clinically meaningful 8–13-point separation between patients who remained stable and those who went on to have cycling. The pattern of findings suggests that illness severity, particularly in regard to depressive symptoms and level of functioning, is an important clinical indicator in predicting which patients are more vulnerable to episode recurrence.
Patients who had stable, nonepisodic courses began their illness at approximately age 20, whereas patients who proved less stable over the course of the year, regardless of cycle frequency, began their illness approximately 3 years earlier, in their midteens. There have now been several studies
(16,
18,
19) in which this relationship has been borne out, and it suggests that early onset of the disease elicits developmental vulnerability to cycling. However, age at illness onset did not prove to be a significant predictor of rapid cycling when more proximal predictors—such as baseline illness severity and recent history of rapid cycling—were taken into account.
A central question in the treatment of bipolar disorder, and more specifically rapid cycling, is whether or not antidepressants are of benefit or if they destabilize the course of illness
(20,
21) . In our study, antidepressant exposure was associated with worse cycling, after we statistically controlled for the severity of baseline depression. In each outcome group, the likelihood of cycling increased linearly with antidepressant use during the first year, such that patients with antidepressant use were more than three times as likely to be rapid cyclers as were patients without such use.
We recognize that our results concerning antidepressant use must be viewed cautiously. First and foremost, patients who received antidepressants were not randomized to treatment. Because of the naturalistic design of the study, we were unable to control for numerous treatment factors, such as antidepressant dose, total time of antidepressant exposure, types of antidepressants used, and the presence of concomitant medications. Thus, we cannot conclude that antidepressants bore a direct causal relationship to increased cycling. Furthermore, antidepressants may have improved the course of bipolar disorder in ways different from those examined here, such as enhancing functioning or quality of life.
Our results can be contrasted with two previous studies by Coryell et al.
(15,
16), both of which indicated that episodes of depression precede rapid cycling, independent of exposure to antidepressants. The authors of these studies concluded that the relationship between antidepressants and phase shifting was associative but not causative. In the present study, we found a relationship between antidepressant use and cycling even after controlling for the baseline level of depression. However, our design did not allow us to control for the level of depression at the time the antidepressants were given.
We failed to find any naturally occurring cutoff point to distinguish patients with four or more episodes from those with two or three episodes per year. Box plots of MADRS, Young Mania Rating Scale, and GAF scores and age at onset of illness revealed a nearly linear relationship between these clinical measures and the number of episodes in the follow-up year. Similar to the cycling pattern in the examination by Kupka et al.
(4), rapid cycling in our study existed along a continuum and suggests that a dimensional, rather than categorical, approach may better conceptualize the phenomenon of frequent cycling.
While a prior history of cycle frequency may have influenced the number of psychiatric visits during the prospective period, both the depression score at study entry and the number of visits independently predicted cycle frequency at follow-up. Thus, patients with more serious illnesses were likely to be seen by psychiatrists more frequently, but the association between illness severity and cycle frequency could not be accounted for by this confound. Nonetheless, researchers examining the phenomenon of rapid cycling should take frequency of observation into account when interpreting results, particularly in naturalistic studies.
Several limitations of our study deserve further comment. The STEP-BD was based at academic, specialty, and tertiary-care sites across the United States, which may have attracted a more select population than might be found in the general community. The population of the STEP-BD was of limited diversity in both race and socioeconomic status, which limits the generalizability of our findings to more diverse populations. Many patients were on complex and changing polypharmacy regimens, and thus the number of medication variables was too great to allow a more in-depth analysis of individual pharmacological treatments and their potential benefits or liabilities. Finally, observation intervals greater than 1 year may be needed to assess whether rapid cycling represents a lifelong vulnerability with poorer prognosis or simply a course modifier that requires longer than 1 year to resolve.
Future studies specifically examining age at onset and its relationship to long-term outcome may be most warranted, as delaying or avoiding the introduction of antidepressants at a young age may enhance the long-term outcome of this treatment-refractory variant. Newer agents, such as quetiapine and lamotrigine, may be increasingly useful, as recent studies have demonstrated their efficacy in treating the depressed phase of both rapid-cycling patients and those with nonrapid cycling
(22,
23) .