Anorexia nervosa, which tends to have an onset during adolescence in females, is characterized by restricted eating, a relentless pursuit of thinness, obsessive fears of becoming fat, and dysphoric mood
(1) . Individuals with restricting-type anorexia nervosa often exercise compulsively, are anhedonic and ascetic, and find little in life that is rewarding aside from the pursuit of weight loss
(1) . Other common but puzzling symptoms include altered judgment and decision making, as illustrated by denial of illness and resistance to treatment. Consequently, anorexia nervosa often has a chronic and relapsing course, with the highest death rate of any psychiatric disorder
(2) . The lack of understanding of the pathophysiology of this illness has hindered the development of effective interventions.
Altered striatal dopamine function may contribute to symptoms in anorexia nervosa. A reduction in CSF dopamine metabolites occurs in malnourished individuals with anorexia nervosa and persists after recovery
(3) . Individuals with anorexia nervosa have altered frequency of functional polymorphisms of dopamine D
2 receptor genes that might affect receptor transcription and translation efficiency
(4) . Individuals with anorexia nervosa show impairment in visual discrimination learning
(5), a task thought to reflect dopamine signaling function. A recent study from our group
(6) provides new clues as to how the dopamine system may be involved in anorexia nervosa symptoms. Patients who had recovered from anorexia nervosa had increased binding of D
2 /D
3 receptors in the anteroventral striatum, a region that contributes to optimal responses to reward stimuli
(7 –
9) . In addition, there were positive correlations between dopamine D
2 /D
3 binding in the dorsal caudate/dorsal putamen and anxiety measures in patients who had recovered
(6) . The anterior ventral striatum and dorsal caudate are components of limbic and executive-associative pathways
(10 –
12) . Thus, striatal dopamine dysfunction might contribute to altered reward and affect, decision making, and executive control, as well as stereotypic motor activity
(11) and decreased food intake
(13) in anorexia nervosa.
The purpose of this study was to assess the response of the anterior ventral striatum to reward and loss in anorexia nervosa. We used event-related functional magnetic resonance imaging (fMRI) to examine the blood-oxygen-level-dependent (BOLD) signal while participants performed a simple choice and feedback task. The task was adapted from a well-characterized guessing-game protocol
(9) known to activate the ventral striatum with a differential response to positive and negative feedback. The Delgado task
(9) was also used to explore the dorsal striatum, because it shows a differential response to reward and punishment in normal subjects.
We examined recovered women to avoid the possible confounding effect of starvation and emaciation. Approximately 50% of individuals with anorexia nervosa eventually recover in the sense that their weight and nutritional status normalize
(14) . However, these individuals often continue to have persistent behavioral symptoms
(15) such as dysphoric mood and obsessionality, as well as altered DA metabolism, as noted above. After recovery, behavioral symptoms tend to be mild compared with when individuals are ill. Such symptoms may be trait markers, since they are often present in childhood, before the onset of anorexia nervosa
(16) . This study focused on women who had recovered from restricting-type anorexia nervosa, since patients with this subtype tend to have the most clear-cut self-denial and ascetic temperament.
Method
Thirteen women who had recovered from restricting-type anorexia nervosa were recruited, as described in a previous publication
(15) . Recovery was defined as maintaining a weight above 85% of average body weight
(17), having regular menstrual cycles, and not having engaged in binge eating, purging, or significant restrictive eating patterns for at least 1 year before the study. Participants had not used psychoactive medication or met criteria for alcohol or drug abuse or dependence, major depressive disorder, or severe anxiety disorder within 3 months of the study. Thirteen age-matched healthy comparison women were recruited through local advertisements. The comparison women (as well as their first-degree relatives) had no history of an eating disorder or any psychiatric, neurological, or serious medical illness. They had been within normal weight range since menarche. The fMRI was performed during the first 10 days of the follicular phase for all participants. This study was conducted according to the institutional review board regulations of the University of Pittsburgh, and all participants gave written informed consent.
Assessments
Axis I diagnoses were made using the Structured Clinical Interview for DSM-IV Axis I Disorders
(18) . Current symptoms were assessed using the State-Trait Anxiety Inventory, Form Y
(19), and the Temperament and Character Inventory
(20) . A modified form of the South Oaks Gambling Screen
(9,
21) identified participants who had excessive gambling behaviors.
Guessing-Game Paradigm
Participants guessed whether a hidden number behind a virtual playing card was greater or less than 5. The range of possible numbers was 1 to 9, with the number 5 excluded. The participant won $2.00 for a correct guess, lost $1.00 for every incorrect guess, and lost $0.50 if she failed to make a guess in the allotted time. At the beginning of each trial, a question mark appeared inside the virtual playing card as a cue for the participant to guess. The question mark remained on screen and responses were accepted for 1 sec. After this, the question mark was replaced by revealing the hidden number for 500 msec immediately followed by a colored arrow (also 500 msec) indicating a win (green up arrow), a loss (red down arrow), or a failure to respond (yellow down arrow). All visual information occurred within the first 2 sec of each trial, followed by an intertrial interval of 12 sec. Participants gave their responses via an ergonomically designed button response unit attached to the right hand. They were told that the computer picked numbers randomly, so that there was no way to know what number was going to be revealed. In actuality, the stimulus control program randomly preselected each trial to be a win or loss trial and presented to the participants a number that was congruent with the outcome. A total of four blocks were run, each consisting of 26 trials (13 wins, 13 losses).
Scanning Procedures
Imaging data were collected with a 3-T Signa scanner (GE Medical Systems). High-resolution anatomical images (1.5 mm
3 ) were acquired for each participant. Additionally, T
1 structural images were acquired with a 3.2-mm thickness (in-plane with the functional images). These had 36 oblique axial slices oriented parallel to the anterior commissure-posterior commissure line, with an in-plane resolution of 0.78 mm
2 and a field of view of 200 mm
2 . Functional images were acquired using a one-shot reverse spiral pulse sequence
(22) with an echo time of 26 msec and a repetition time of 2000 msec (one full volume scan acquired every 2 sec); 30 oblique axial slices were acquired with an in-plane resolution of 64×64 with 3.125 mm
2 pixels and a slice thickness of 3.2 mm, with a field of view of 200 mm
2 . Seven functional volumes were acquired per trial.
fMRI Data Processing and Analysis
The preprocessing for both the regions-of-interest and the voxelwise analysis was the same. Tools included NeuroImaging Software (NIS; http://kraepelin.wpic.pitt.edu/nis/), Automated Image Registration (AIR;
23 ), and Analysis of Functional NeuroImages (AFNI;
24 ). fMRI data were preprocessed with slice-time correction (AFNI-3dtshift), motion correction using 6-parameter algorithm (AIR), high-pass filtering (AFNI-3dfourier, 0.024 Hz), a between-block baseline correction, and a within-block linear detrend (NIS, 4 SD correction for outliers). Each participant’s brain was registered to the Montreal Neurological Institute reference brain (ftp://ftp.mrc-cbu.cam.ac.uk/pub/imaging/Colin/) using a 60-parameter warp function (AIR). Lastly, the functional data were smoothed using a three-dimensional Gaussian filter, 6.25×6.25×6.4 mm full width at half maximum.
The primary regions-of-interest analysis focused on the caudate and ventral striatal regions previously identified in healthy comparison subjects by Delgado et al.
(9,
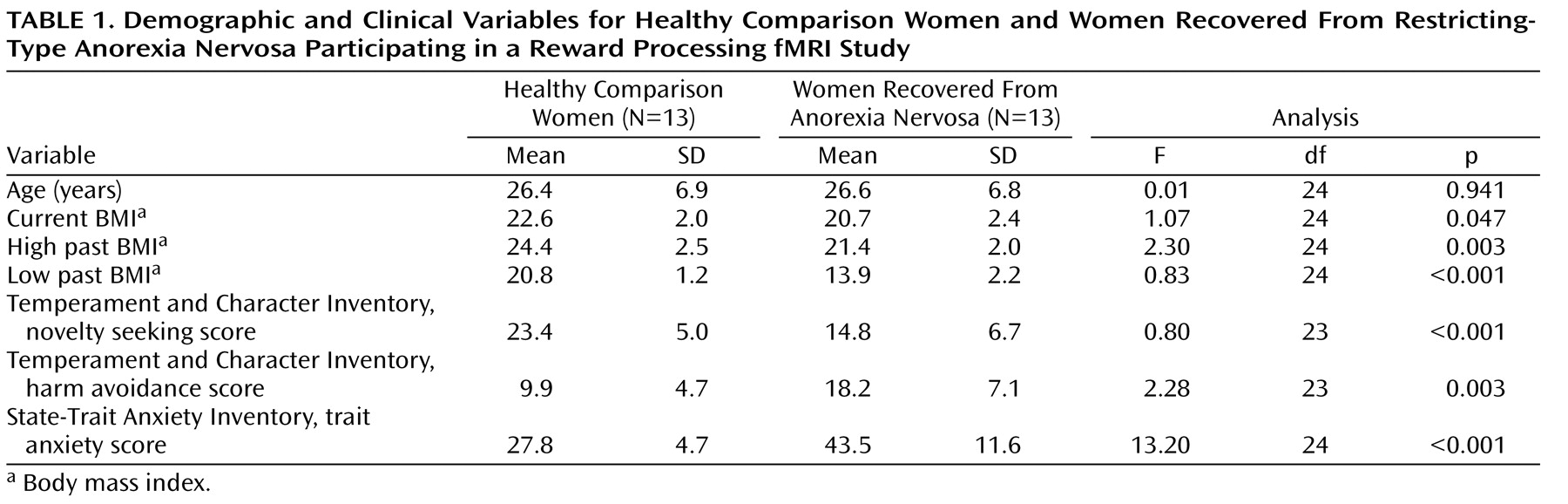
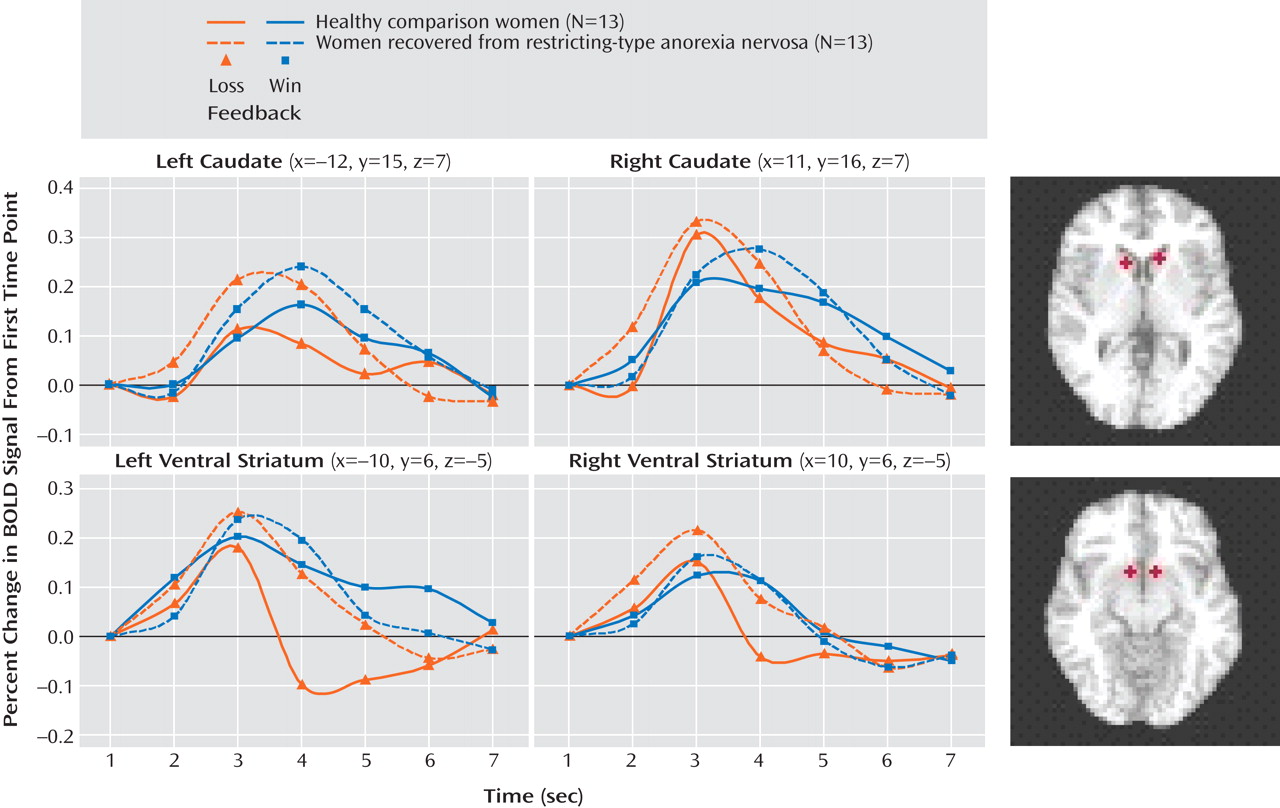
25) for this task. The Talairach coordinates for three striatal regions were as follows: right caudate, –12, 15, 7; left caudate, 11, 16, 7; and left ventral striatum, –10, 6, –5. We also included the right ventral striatum (10, 6, –5). We extracted the mean signal in a 3.2-mm radius (1 voxel radius) sphere centered at these Talairach coordinates. The time course of the BOLD response within each region of interest was transformed to a percentage change from baseline (T01) for visual inspection and interpretation. The raw extracted time-course data from these regions of interest were subjected to follow-up tests in order to specify effects. The time-series were subjected to a three-way analysis of variance (ANOVA) with group as a between-subjects factor (two levels), outcome (win or loss) as a within-subjects factor (two levels), and time (time points T01–T07) as a repeated-measures factor (seven levels).
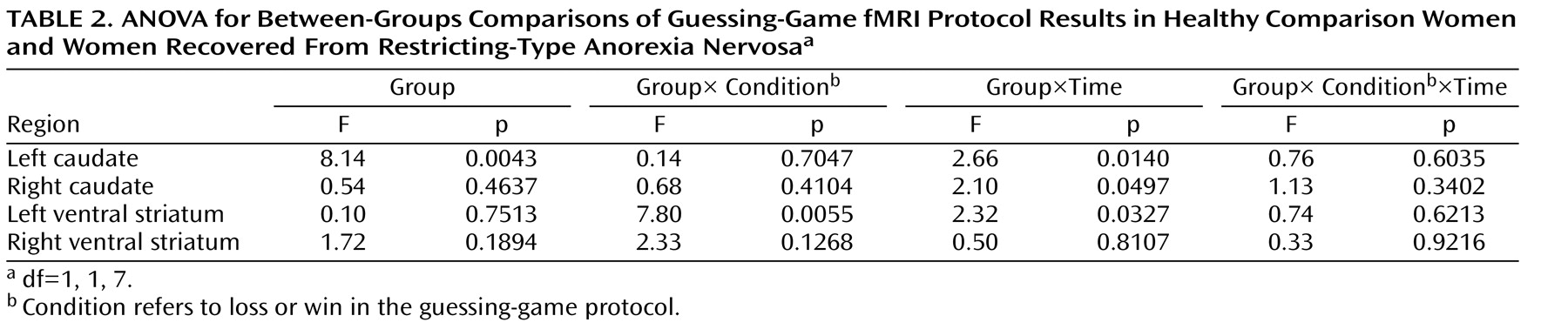
A secondary full-brain voxelwise analysis was performed with the NIS package, relying on ANOVA. Initially, each group was examined separately using ANOVA and a three-way ANOVA with group as a between-subjects factor (two levels), with outcome as a within-subjects factor (two levels), and with time (time points T01–T07) as a repeated-measures factor (seven levels). Each test was thresholded according to a per-voxel p value and a contiguity requirement. Together, the p value and contiguity thresholds defined clusters of activation.
Clinical behavioral data analyses were conducted using SPSS, version 12.0, and SigmaStat, version 2.03. The two groups were compared with an independent-samples t test.
Discussion
This is the first study to show that individuals who have recovered from restricting-type anorexia nervosa have altered patterns of response in the ventral and dorsal striatum to positive and negative feedback. As in the Delgado et al. study
(9), an anterior ventral striatum response that distinguished between winning and losing was seen in the comparison women and not in the anorexia nervosa group. These findings suggest that individuals with anorexia nervosa may have difficulty discriminating between positive and negative feedback, relative to healthy comparison subjects. Similarly, in a study using a startle reflex paradigm
(26), a generalized failure to activate the appetitive motivational system was observed in individuals with anorexia nervosa. Increased anterior ventral striatum dopamine D
2 /D
3 receptor binding in recovered women could contribute to this alteration in anterior ventral striatum function
(6) . Animal studies show that the anterior ventral striatum depends on dopamine to process motivational aspects toward stimuli by modulating the influence of limbic inputs on striatal activity
(7,
8,
11,
27) . Dopamine is thought to mediate the “binding” of hedonic evaluation of stimuli to objects or acts (“wanting” response)
(28) . Even abstract proxies for reward (money) cause activations in the anterior ventral striatum in proportion to the reward amount or the deviation from an expected payoff
(7) . Individuals who have recovered from anorexia nervosa have long been noted to be anhedonic and ascetic, able to sustain self-denial of food as well as of most comforts and pleasures in life. Such a temperament persists, in a more modest form, after recovery
(15,
29) . These anterior ventral striatum responses to cues may reflect a failure to appropriately bind, scale, or discriminate responses to salient stimuli. These data support the possibility that individuals who have recovered from anorexia nervosa may have an impaired ability to identify the emotional significance of stimuli
(10) and may be important in understanding why it is so difficult to motivate individuals with anorexia nervosa to engage in treatment or appreciate the consequences of their behaviors
(30) .
In contrast, the recovered women had exaggerated caudate-dorsal striatum activation in the regions-of-interest analysis. The caudate nucleus is activated by those tasks in which there exist both a perceived connection between action and outcome and some uncertainty about whether the action will lead to the desired outcome
(31) . O’Doherty and colleagues
(32) postulated that dopamine projections to the dorsal striatum might be involved in the modulation of stimulus-response or stimulus-response-reward associations, corresponding to the instrumental actor. The “actor” maintains information about the rewarding outcomes of actions to enable better ones to be chosen more frequently. Perhaps greater activation of this region in the recovered women indicates an attempt at “strategic” (as opposed to hedonic) responses to improve the ratio of wins to losses. In the absence of appropriate reward processing via ventral striatum/dopamine paths, individuals who have recovered from anorexia nervosa may focus on a detailed strategy rather than the overall phenomenon (attempting to find strategies within the guessing game). This hypothesis is supported by the results of a posttask questionnaire in which seven recovered women identified a strategy in target selection, whereas none of the comparison women did so (data not shown). Neurocognitive studies show that individuals with anorexia nervosa have an enhanced ability to pay attention to detail or use a logical-analytic approach but exhibit worse performance for global strategies (
33 ; C. Lopez et al., unpublished 2006 data).
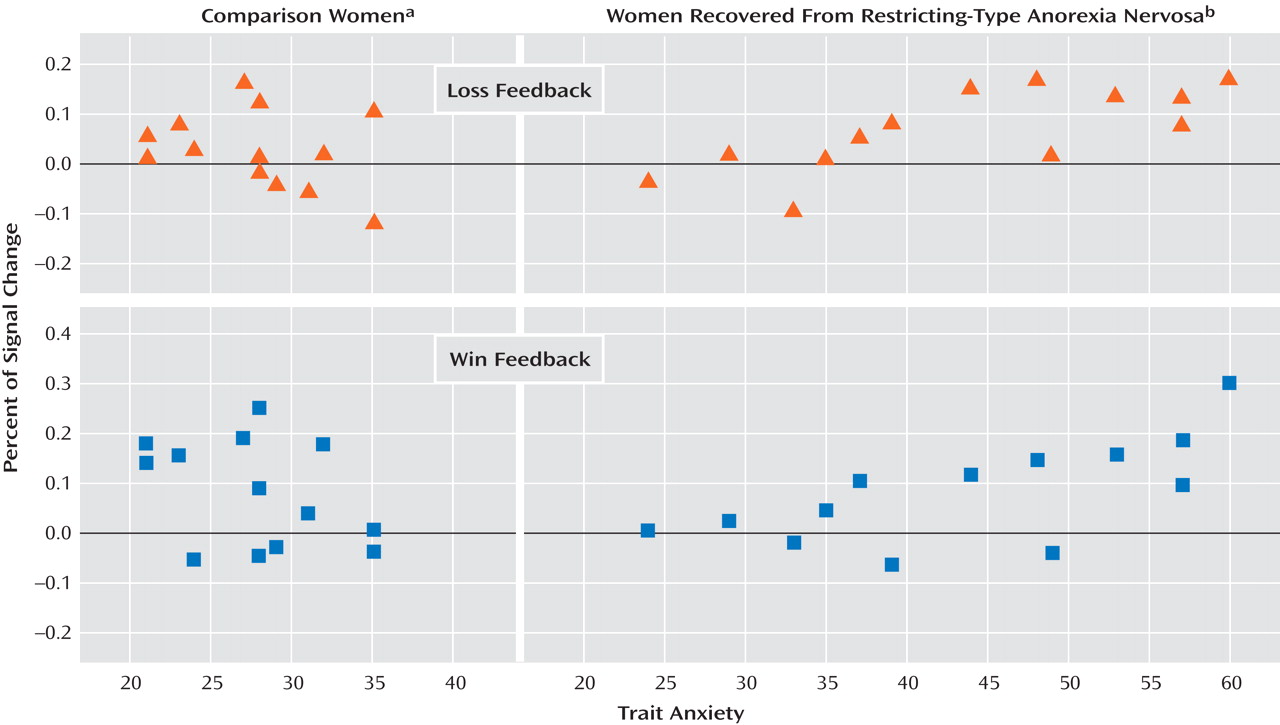
Only in the recovered women was a significant positive relationship observed between baseline trait anxiety and the percentage change in hemodynamic response to wins or loses in the left caudate (in the right caudate, the difference approached but did not achieve significance). The most anxious individuals with anorexia nervosa may respond in an overly “cognitive” manner to both negative and positive stimuli and consequently may not be able to process information about rewarding outcomes of action
(31,
32) . Thus, performance—that is, being right or wrong—may become more important than hedonic rewards. Several “cognitive” cortical regions, including the dorsolateral prefrontal cortex and parietal cortex, which project to the dorsal caudate
(12,
34), had greater activation in the recovered women than in the comparison women (see the tables in the online supplement). Recruitment of the dorsolateral prefrontal cortex and parietal-striatal activation in the recovered women may reflect recruitment of conscious or unconscious strategies to locate the win target, in contrast to registering the hedonic value of the win by anterior cingulate-ventral striatal activation, as seen in the comparison women. Similarly, dorsal caudate dopamine D
2 /D
3 receptor binding in this striatal region
(6) was positively correlated with anxiety or harm avoidance, a trait characterized by anticipatory worry and fear of uncertainty. Phasic activation of dopamine neurons changes with reward probability, while slower, more sustained activations of dopamine neurons are related to reward uncertainty
(35) . Whether exaggerated caudate response is related to oversensitivity to uncertainty remains to be determined.
Are these findings specific to individuals who have recovered from anorexia nervosa, or are they related to comorbid anxiety, depression, or obsessive-compulsive disorder? fMRI studies using a variety of reward paradigms have reported dysfunction of several striatal regions in depression, anhedonia, anxiety, and obsessive-compulsive disorder
(36 –
38) . As noted above, a dysphoric obsessive temperament is common in individuals with anorexia nervosa; its onset tends to be in childhood before the onset of anorexia nervosa
(16), and it persists after recovery
(15,
29) . Thus, such a temperament may be part of the vulnerability to developing anorexia nervosa. Perhaps disorders of disturbed affective and impulse regulation involve aberrant limbic-striatal pathways
(10) but with different patterns on a molecular level.
Our major focus in this study was to use regions-of-interest analysis to evaluate the striatum. We were aware of the possibility that group effects would be confounded by differences in overall fMRI baseline signal, so we focused on interaction analyses that depend on condition or time to make those influences unlikely. We also included an exploratory voxelwise analysis to assess the entire circuit. Striatal differences were found between the comparison women and the recovered women in the voxelwise analysis, although of a somewhat different nature, and they may have been compromised by our relatively small sample size. The recovered women had a lower current BMI than the comparison women, as is often described in comparable data sets
(29) . fMRI group analyses may in theory be confounded by differences in neuroanatomy between the groups, which can lead to inconsistent alignment of the fMRI data into a common anatomical space. However, a recent study from our group
(39) that included some of these participants showed normal brain volume after recovery from anorexia nervosa. Still, it is possible that the brains of those recovered from anorexia nervosa systematically have more alignment error than those of healthy individuals. Finally, the cross-sectional design of this study does not allow us to determine whether these findings are a trait that contributes to the onset of anorexia nervosa or a “scar” that is the consequence of past malnutrition and weight loss.
Conclusions
Do individuals who have recovered from anorexia nervosa have an imbalance between pathways that identify the emotional significance of environmental stimuli
(10) and pathways responsible for the performance of planning and effortful functions? In response to the guessing task, the comparison women in this study appropriately activated anterior ventral striatum reward pathways and responded to wins and losses by “living in the moment,” that is, they made a guess and then moved on to the next task. People with anorexia nervosa do not live in the moment. They tend to have exaggerated and obsessive worry about the consequences of their behaviors, looking for rules when there are none, and they are overly concerned about making mistakes. The present results suggest that these cognitive interferences may have neural correlates. In contrast to healthy individuals, they have greater activation of dorsal caudate and do not differentiate between reward and punishment in anterior ventral striatum limbic circuits. Thus, individuals who have recovered from anorexia nervosa may be less able to precisely modulate affective response to stimuli but have increased traffic in neurocircuits concerned with planning and consequences.