Eating disorders are an important cause of physical and psychosocial morbidity in adolescent girls and young adult women, while they are much less frequent in men. These disorders are characterized as a primary disturbance of eating habits or weight-control behavior resulting in significant impairment of physical health or psychosocial functioning. According to DSM-IV, the diagnostic group of anorexia nervosa is defined by the refusal to maintain body weight at or above a minimal normal weight. This is combined with an intense fear of gaining weight or becoming overweight, with a disturbance in the way in which body weight or shape is experienced, and with amenorrhea. Anorexia nervosa is divided into the following two types: 1) the restricting type, in which patients primarily restrict their eating, and 2) the binge eating/purging type, in which patients are regularly engaged in binge eating or purging behavior
(1) . Nevertheless, there is frequently a combination of symptoms over time, and a number of patients with anorexia nervosa develop bulimia nervosa or an atypical eating disorder.
The brain regions critically involved in the pathophysiology of anorexia nervosa are a question of continuing debate. This lack of decisive knowledge about the locus of anorexia nervosa-related changes in the brain has not only hampered the investigation of the mechanisms leading to anorexia nervosa but also approaches to more successful treatment.
Various lines of imaging studies have aimed to identify the brain regions critically involved in the pathophysiology of anorexia nervosa. A systematic review of lesion studies on patients who developed an eating disorder subsequent to cerebral damage concluded that “complex syndromes, including characteristic psychopathology of eating disorders, are associated with right frontal and temporal lobe damage”
(2) . In studies that used single photon emission computed tomography (SPECT), anorexia nervosa patients showed hypoperfusion in the medial prefrontal cortex and anterior cingulate cortex
(3,
4), even after weight restoration
(5) . Using functional magnetic resonance imaging (fMRI), patients with restricting anorexia nervosa, who were either recovered or chronically ill, were compared with healthy comparison subjects with respect to brain activation in response to food stimuli. This study revealed increased medial prefrontal and anterior cingulate cortex activation as well as a lack of activity in the inferior parietal lobule in the recovered group relative to the healthy comparison group. The comparison of the recovered group with chronically ill patients showed increased activation of the right lateral prefrontal, frontopolar, and dorsal anterior cingulate cortices
(6) . In another fMRI study, nonrecovered patients suffering from either bulimia or anorexia nervosa were confronted with food stimuli
(7) . Patients with both eating disorders also displayed increased activation in the anterior cingulate cortex
(7) . Studies using positron emission tomography (PET) with serotonin (5-HT )-specific radioligands have implicated alterations of 5-HT
1A and 5-HT
2A receptors and the 5-HT transporter in the frontal, cingulate, temporal, and parietal cortices
(8), which persist after physical recovery.
Another approach to identify the brain regions critically involved in the pathophysiology of anorexia nervosa constitutes the analysis for structural brain changes. In recent years, morphometric techniques have been developed that allow the localization of subtle gray matter changes at the group level. One of these techniques, voxel-based morphometry, is based on high-resolution magnetic resonance imaging (MRI). Although the technique reveals alterations in the local concentration of gray matter, several studies have demonstrated that these structural changes are directly related to functional changes in brain activity
(9,
10) . However, applying voxel-based morphometry in anorexia nervosa requires caution, since at low weight global changes in the brain structure exist that are commonly attributed to malnutrition
(11 –
14) .
Against this background, we applied voxel-based morphometry in recovered anorexia nervosa patients to study both global and regional structural brain changes.
Method
Sample Collection
The present study was approved by the local ethics committee. Since anorexia nervosa occurs much less frequently in men than in women and it is unclear whether the pathophysiology of the disorder in men equals that in women
(15), we examined female anorexia nervosa patients exclusively. We examined recovered patients, since we expected the global brain tissue decrease at low weight to conceal region-specific gray matter changes. Recovery was defined by a body mass index (i.e., weight in kilograms divided by the square of height in meters) above 17.0 kg/m
2 and regular menstrual cycles for at least 6 months. Participants were recruited from three therapy centers for eating disorders. We included 22 patients. For the patient group, the following inclusion criteria were predefined: between 18 and 48 years of age, anorexia nervosa restricting type during the first year of the disease (according to DSM-IV), and exclusion of comorbidity. The following lifetime diagnoses were predefined as exclusion criteria: posttraumatic stress disorder (PTSD), manic episodes, schizophrenia, obsessive-compulsive disorder (OCD), substance use disorders, and borderline personality disorder. The diagnosis of major depressive episodes only constituted an exclusion criterion when it occurred not only at times of low weight but also at times of recovery. We used the diagnostic items of a modified version of the Structured Interview for Anorexic and Bulimic Disorders for DSM-IV and ICD-10
(16) . All patients who were included fulfilled the diagnostic criteria for anorexia nervosa in the past but not at the time of scanning. PTSD, manic or major depressive episodes, schizophrenia, OCD, and substance use disorders were excluded by international diagnosis checklists according to DSM-IV
(17) . Borderline personality disorder was ruled out by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID II)
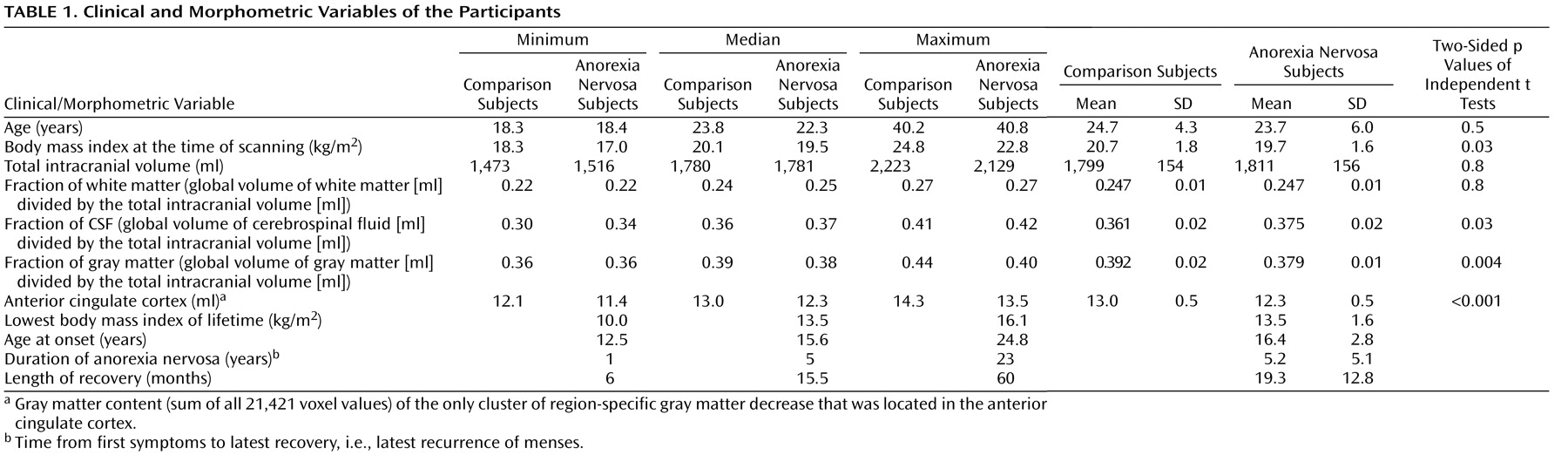
(18) . Only patients who were likely to fulfill the inclusion criteria according to their patient records were asked by their therapists in writing whether they were willing to participate in a telephone interview with the aim to identify appropriate anorexia nervosa patients for an imaging study. Fifty-one patients agreed to participate in the interview by completing a form accordingly and sending it to the imaging center. Among the patients interviewed, 25 were invited to participate in the study. After complete description of the study to the subjects, written informed consent was obtained. Prior to scanning, the subjects were interviewed in detail by an experienced investigator who was specifically trained to use the diagnostic tools applied. This resulted in the identification of psychiatric comorbidity in two patients and, hence, to their exclusion from the study. To further characterize the anorexia nervosa patients, the following parameters were required: body mass index at the time of scanning, lowest body mass index of lifetime, age at onset (i.e., when anorexia nervosa symptoms resulted in significant impairment of physical health or psychosocial functioning for the first time), duration of anorexia nervosa (i.e., time from anorexia nervosa onset to latest recovery defined by latest recurrence of menses), and length of recovery (i.e., time from latest recurrence of menses).
After providing written informed consent, all comparison women were interviewed as described above and only included when there was no indication of any psychiatric disorder in their lifetime.
MRI Acquisition
Two MRI sequences were acquired from every participant using the same scanner (Siemens Magnetom Symphony; magnetic field intensity, 1.5 Tesla). For voxel-based morphometry, sequence 1 was used (sequence=T1 MPRAGE; plane=sagittal; number of slices=160, slice thickness=1 mm, voxel size=1×1×1 mm 3, flip angle=15°; field of view=256×256 mm, time to repeat=8.9 msec, echo time=3.93 msec, T1=800 msec). Sequence 2 was used to support the detection of abnormal or unusual findings (sequence=fluid attenuated inversion recovery; plane=axial; slice thickness=6 mm). Apart from one patient who was excluded from the study because of very large artifacts, probably resulting from her dental braces, neither abnormal nor unusual findings were detected.
Voxel-Based Morphometry: Preprocessing
Voxel-Based Morphometry 2 software (http://dbm.neuro.uni-jena.de/vbm), an extension of Statistical Parametric Mapping (SPM)2 software (http://www.fil.ion.ucl.ac.uk/spm), was applied. Voxel-Based Morphometry 2 applies the “optimized” protocol and a hidden Markov random field model
(19) . We used study-specific prior probability maps and a Gaussian kernel of 14 mm for smoothing.
Calculation of Global Volumes
Gray matter, white matter, and CSF were derived from the non-normalized segmented images as provided by SPM2 after the first segmentation process. Total intracranial volume was approximated by the sum of the global volumes of gray matter, white matter, and CSF. To correct for head size, brain tissue fractions of gray matter, white matter, and CSF were calculated by dividing the respective volumes by the total intracranial volume.
Analysis of Global Volumes
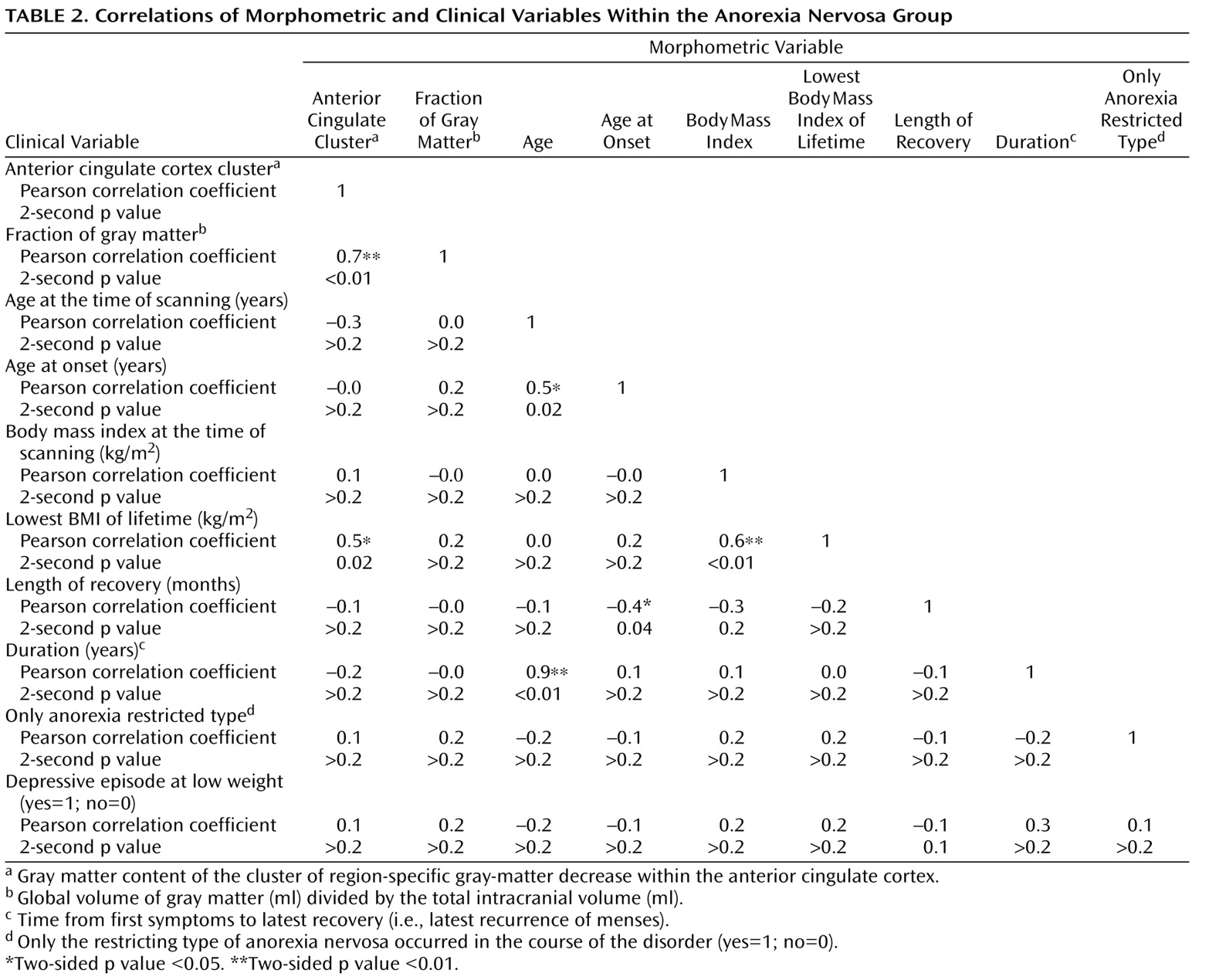
For group comparisons of global values, two-sided independent t tests were performed using standard software (SPSS, version, 14.0.1). Within the anorexia nervosa group, the following correlation analyses were performed: fraction of gray matter with the duration of anorexia nervosa, age of first diagnosis, length of recovery as well as lowest body mass index of lifetime, and body mass index at the time of scanning. Moreover, a univariate analysis of variance (ANOVA) was performed with fraction of gray matter as a dependent variable and the parameters mentioned above as independent variables.
Voxel-Based Morphometry: Statistical Analyses
We included only voxels with a gray matter value greater than 0.2 (maximum value=1) and greater than both the white matter and CSF values to analyze only voxels with sufficient gray matter and to avoid possible edge effects around the border between gray matter, white matter, and CSF. Because the analysis of fraction of gray matter revealed significant differences, we performed two voxel-by-voxel analyses of covariance (ANCOVA). In the first ANCOVA, only age was included as a confounding covariate (to remove variance explained by age). We will refer to this approach as “analysis for the regional distribution of gray matter changes.” In the second ANCOVA, age and fraction of gray matter values were included as confounding covariates in order to exclusively identify changes that cannot be explained by global effects. We will refer to this approach as “analysis for region-specific gray matter changes.” Within the anorexia nervosa group, the following correlation analyses were performed: gray matter content (sum of all voxel values) of the clusters of region-specific gray matter changes with lowest body mass index of lifetime, body mass index at the time of scanning, duration of anorexia nervosa, age at the time of scanning, age at first diagnosis, length of recovery, fraction of gray matter, occurrence of major depressive episodes at low weight (no=0; yes=1), and occurrence of an eating disorder other than the restricting type of anorexia nervosa in the course of the disorder (no=0; yes=1). Moreover, an ANOVA was performed with gray matter content of the clusters of region-specific gray matter changes as a dependent variable and the parameters mentioned above as independent variables.
We applied a height threshold of p<0.05, corrected for all voxels included in the analysis applying the family-wise error
(20) . To indicate the extension of gray matter changes, significant clusters were displayed at a voxel threshold of p<0.01.
Discussion
This study aimed at identifying both global and regional structural brain changes in recovered anorexia nervosa patients. First, we analyzed our data for global changes and found that global gray matter (i.e., fraction of gray matter) but not white matter was significantly decreased in the anorexia nervosa group. This finding complies with the results of two previous longitudinal studies
(13,
14) . In these studies, patients were scanned at low weight and displayed decreased global gray and white matter. In the patients who underwent rescanning after recovery, only global gray matter remained significantly lower. By contrast, Swayze et al.
(11,
12) found only reduced global white matter but not gray matter in anorexia nervosa patients at low weight relative to comparison subjects, although follow-up scans of the anorexia nervosa patients revealed significant increase in both global white and gray matter. Another cross-sectional MRI study
(21) found normal brain tissue volumes after long-term recovery in anorexia nervosa patients who were slightly less affected than our patients (lowest body mass index of lifetime: 14.1 [SD=1.4] versus 13.5 [SD=1.6]). In addition, the mean length of recovery was longer (28.7 months versus 19.3 months) and the body mass index at the time of scanning was higher (21.1 [SD=2.0] versus 19.7 [SD=1.6]) in this group of patients than in our anorexia nervosa group. This raises the question of whether restoration of brain morphology was incomplete in our anorexia nervosa group at the time of scanning. If this were the case, the global gray matter should have increased with the length of recovery, but our correlation analysis does not support this assumption (
Table 2 ). Conversely, we did not find a clear association of global gray matter with any of the clinical variables, including the severity of anorexia nervosa estimated with the lowest body mass index of lifetime and the duration of anorexia nervosa (
Table 2 ). Another question that emerges from our data is whether functional deficits can be assumed merely because of a global gray matter decrease of approximately 1% at the group level. This seems very unlikely, since there are no compelling links between brain size and behavioral capacity within the range of brain size observed in our study groups
(22) . However, our data do not exclude the possibility of specific cognitive impairments in anorexia nervosa, which is still a matter of debate
(23,
24) but beyond the scope of the present study.
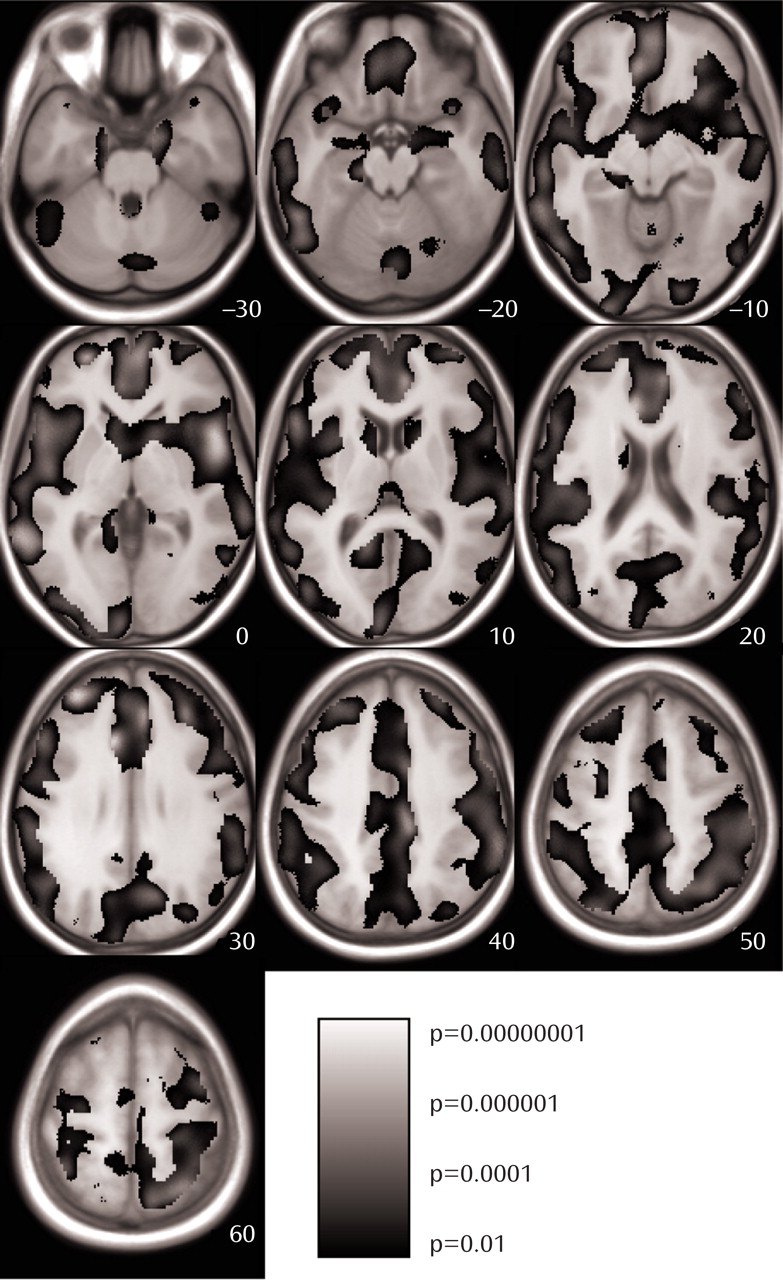
Second, we analyzed our data for the regional distribution of gray matter changes and found that gray matter loss spread across the whole brain (
Figure 1 ), indicating that the magnitude of gray matter loss is generalized rather than specific to particular brain regions.
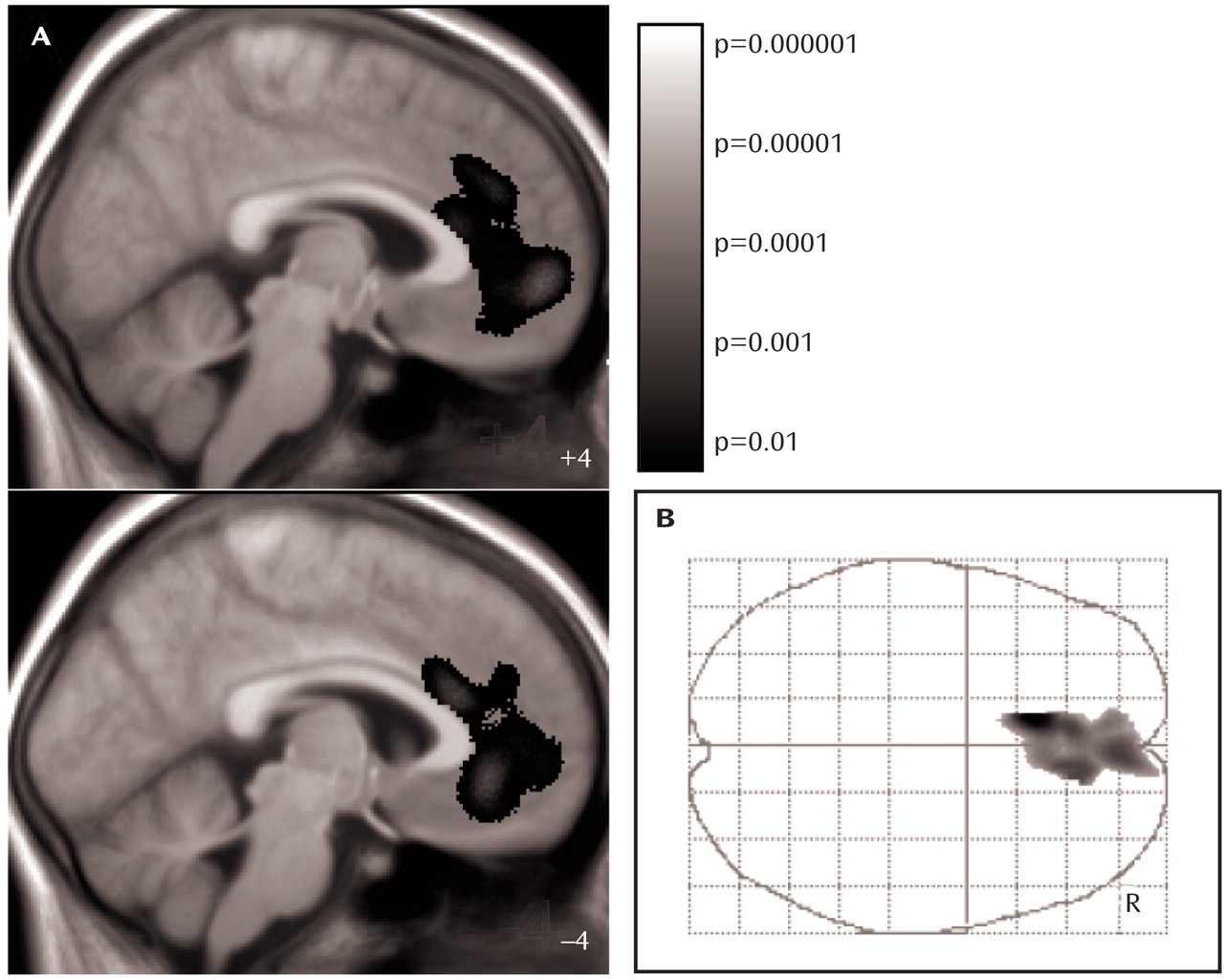
Third, we searched for region-specific gray matter changes (i.e., regional changes that cannot be explained by global gray matter decrease) by additionally including fraction of gray matter as a confounding covariate in the ANCOVA model. In this analysis, only one small cluster of gray matter loss within the left anterior cingulate cortex was identified. Since both the ANCOVA-model applied and correction for multiple comparisons with the family-wise error
(20) constitute very conservative approaches, we displayed this result at a voxel level of p<0.01. To exclude that the enlargement of this cluster at this liberal threshold occurred by chance, we considered the significance at the cluster level (corrected p value=0.0002). Moreover, we analyzed the gray matter content of the anterior cingulate cortex cluster for a correlation with several clinical variables. We used the lowest body mass index of lifetime to estimate the severity of anorexia nervosa and not the duration of anorexia nervosa, since this parameter was correlated with the age of our patients (
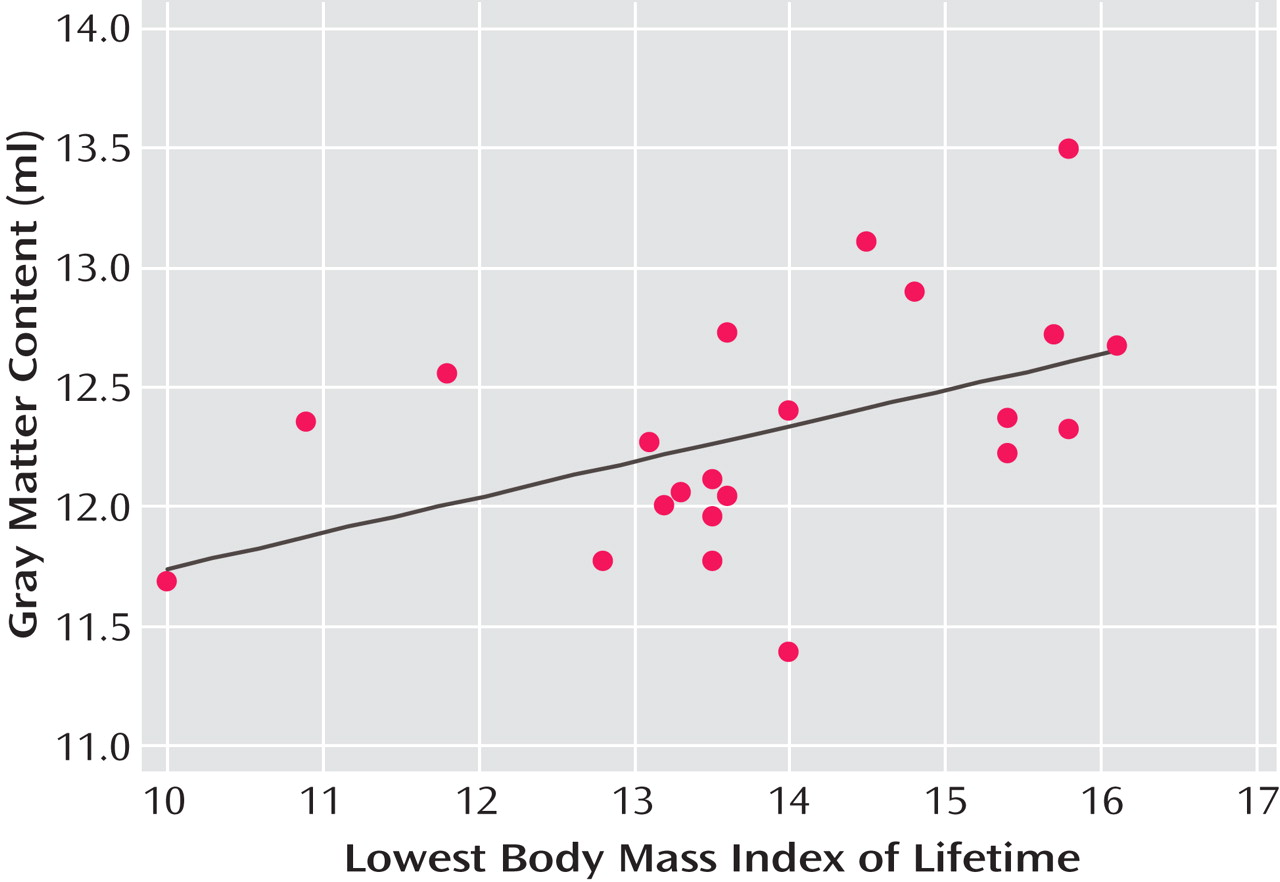
Table 2 ). Notably, the severity of anorexia nervosa was the only clinical variable that correlated with the gray matter content of the anterior cingulate cortex cluster (
Figure 3,
Table 2 ). This effect was robust and remained significant after correction for global gray matter volume and the remaining clinical variables. These findings allow for two interpretations. Either gray matter decrease within the anterior cingulate cortex is an effect of anorexia nervosa, indicating that the anterior cingulate cortex is more vulnerable than the rest of the brain to conditions associated with anorexia nervosa such as malnutrition, or gray matter decrease within the anterior cingulate cortex is a cause of anorexia nervosa, indicating that the anterior cingulate cortex plays an original role in the pathophysiology of the disorder. We are not aware of scientific data indicating a particular vulnerability of the anterior cingulate cortex. In contrast, given the complex clinical picture of anorexia nervosa, it seems well conceivable that the functionally heterogenic structure of the anterior cingulate cortex plays an important role in the pathophysiology of this eating disorder.
In the 19th century, Broca already postulated that the anterior cingulate cortex integrates internal drives and emotions with higher cognitive functions
(25) . Today, the anterior cingulate cortex is regarded as a pivotal component of circuits that underlie functions such as attention, learning, language, and motor behavior
(26) . There are at least three functional subdivisions of the anterior cingulate cortex, of which the first two (rostral affective/visceral region and dorsal cognitive region) overlap with our anterior cingulate cortex cluster. The rostral affective/visceral region is located inferior and anterior to the genu of the callosum. Strongly interconnected with the orbitofrontal cortex and amygdala, this region is not only involved in autonomic and endocrine functions but also in higher-order functions such as conditioned emotional learning, assessments of motivational content, and assigning emotional valence to internal and external stimuli. The dorsal cognitive region, the site of the peak voxel of the anterior cingulate cortex cluster, lies superior to the callosum, has extensive reciprocal connections with temporal and other frontal areas, and participates in response selection and cognitively demanding information processing
(27,
28) .
Evidence for an original role of the anterior cingulate cortex in the pathophysiology of anorexia nervosa derives not only from our structural data but also from other imaging studies. Applying SPECT, anorexia nervosa patients showed hypoperfusion in the anterior cingulate cortex
(3 –
5), indicating an impaired or inhibited activity of this region. A PET study with specific radioligands was performed to localize alterations of the serotonin system and, notably, identified the anterior cingulate cortex
(8) . Further, fMRI studies indicate altered activation in the anterior cingulate cortex of anorexia nervosa patients specific to food stimuli
(6,
7) . In summary, converging evidence suggests that in anorexia nervosa the anterior cingulate cortex is altered with regard to both structure and function. However, we are far from arriving at a unifying model for the pathophysiology of anorexia nervosa, given the fact that the anterior cingulate cortex constitutes a multimodal region where complex phenomena, such as emotion, internal drives, reward seeking, attention, higher cognitive function, and learning, are functionally integrated. Moreover, some results of the imaging studies described above seem contradictory (e.g., hypoperfusion versus hyperactivation), and a considerable proportion of the brain regions that were additionally identified do not correspond. Yet, these data indicate that the pathophysiology of anorexia nervosa can almost certainly not be reduced to just a dysfunction of the anterior cingulate cortex. In addition, morphometric changes of the anterior cingulate cortex have also been reported in further psychiatric disorders such as OCD
(29), PTSD
(30), and schizophrenia
(31), and thus it remains open for study whether changes of the anterior cingulate cortex in various psychiatric disorders result from common root causes of these disorders or from the involvement of different functional subdivisions of the anterior cingulate cortex.
Our study has some methodological limitations. Since recovery of our patients might have been incomplete and since we did not examine morphological changes longitudinally, the conclusion regarding the reversibility of global gray matter decrease remains speculative. Although we could show that region-specific gray matter decrease in the anterior cingulate cortex is directly related to the severity of anorexia nervosa, it remains to be elucidated whether this abnormality is a vulnerability marker and predictor (of relapse or chronicity) of anorexia nervosa. Correlation analyses of structural brain changes with the genetic load derived from anorexia nervosa patients with numerous siblings or from genetic testing
(32) constitute a possible approach regarding the question of whether gray matter decrease in the anterior cingulate cortex results from genetic or environmental factors. Last, further neuroimaging studies are warranted to assess the anterior cingulate cortex in anorexia nervosa patients with regard to both structure and function.