Abnormalities in inhibitory networks have been hypothesized to be a shared and transdiagnostic pathophysiological substrate of disruptive behavior disorders
(1) . Attention deficit hyperactivity disorder (ADHD), characterized by behavioral features of inattention, impulsiveness, and hyperactivity (DSM-IV, reference
1 ) has consistently been associated with neuropsychological deficits in inhibitory functions
(2,
3) and with brain abnormalities in the inhibition-mediating inferior and dorsolateral prefrontal cortex, the cingulate, and the caudate
(2,
4 –
8) .
Conduct disorder, a disorder of proactive aggression and antisocial behavior, overlaps clinically, behaviorally, and cognitively with ADHD, and a high comorbidity exists between both disorders
(9) . Uncertainty about the distinction between the disorders has led to clinical difficulties expressed in differences in diagnostic schemes: in the DSM-IV, they are treated as independent disorders; in the ICD-10, the mixed state is regarded as a subtype of hyperkinetic disorder
(10) . Furthermore, it has been uncertain whether conduct disorder has a neurobiological basis other than that associated with ADHD
(11) . A dysfunction in inhibitory networks may be a distinctive neurocognitive basis of conduct disorder. Deficits in frontal-temporal/limbic inhibitory mechanisms have been suggested to account for the lack of control over aggressive and antisocial behaviors
(12) . Neuropsychological studies show that children with conduct disorder/oppositional defiant disorder alone are also impaired in their inhibitory capacity
(13) . Although findings are less consistent with smaller effect sizes
(14) and in comorbid cases, ADHD symptoms account more for poor inhibitory performance than symptoms of oppositional deviant disorder/conduct disorder
(15) .
Electrophysiological studies show that children with conduct disorder/oppositional defiant disorder alone differ from comparison subjects in their frontal- and parietal-evoked potentials during attention and inhibition tasks
(1,
16,
17) but not from a pure ADHD group
(1,
16) . The only functional magnetic imaging (fMRI) study of children with conduct disorder comorbid with ADHD found abnormal anterior cingulate activation in relation to comparison subjects during emotion processing
(18), and there is evidence for structural abnormalities in the temporal lobes
(19) . No fMRI study to date, however, has investigated the neurofunctional substrate of inhibitory control in patients with conduct disorder or how it differs from that of ADHD patients. Modern functional neuroimaging will be an important aid in the differentiation of clinically similar disorders if it can identify differences in the objectively measurable pathophysiological mechanisms or “biomarkers” that underlie overlapping behavior features.
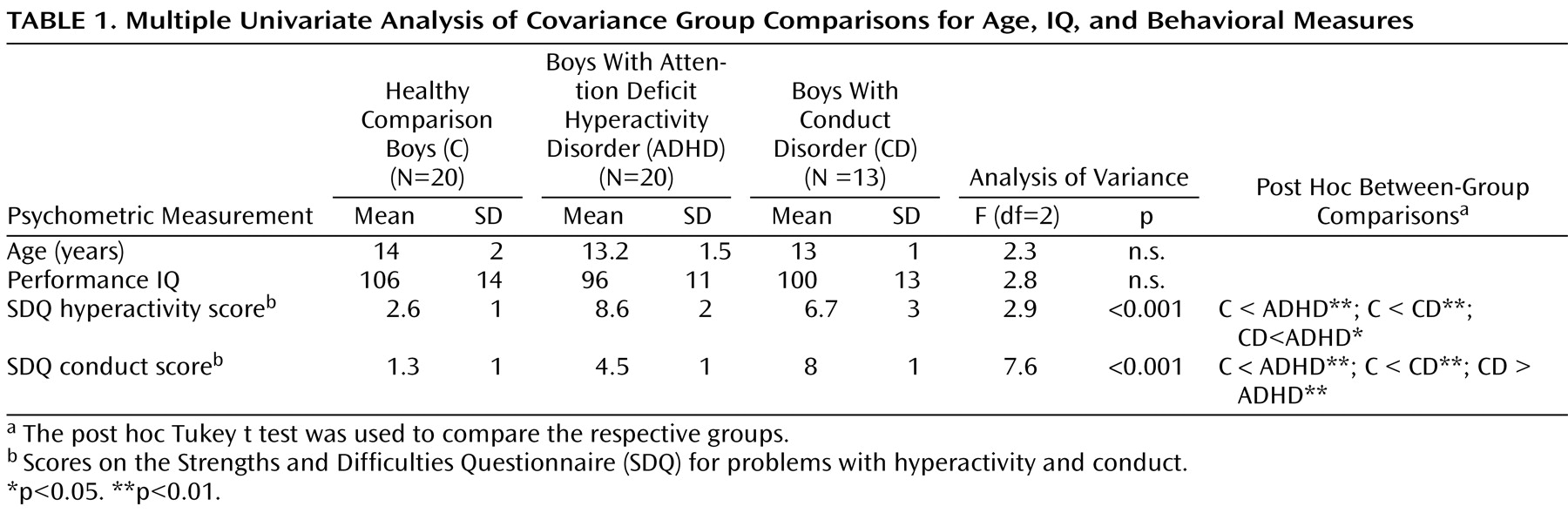
The aim of this study was to use fMRI to investigate the differences and commonalities in the neurobiology of inhibitory control in a carefully selected pediatric patient group meeting criteria for noncomorbid conduct disorder compared to healthy adolescents and to adolescents with noncomorbid ADHD. For this purpose, we used an individually adjusted stop task that controls for performance differences and measures the neural correlates of successful inhibition and inhibitory failure.
Discussion
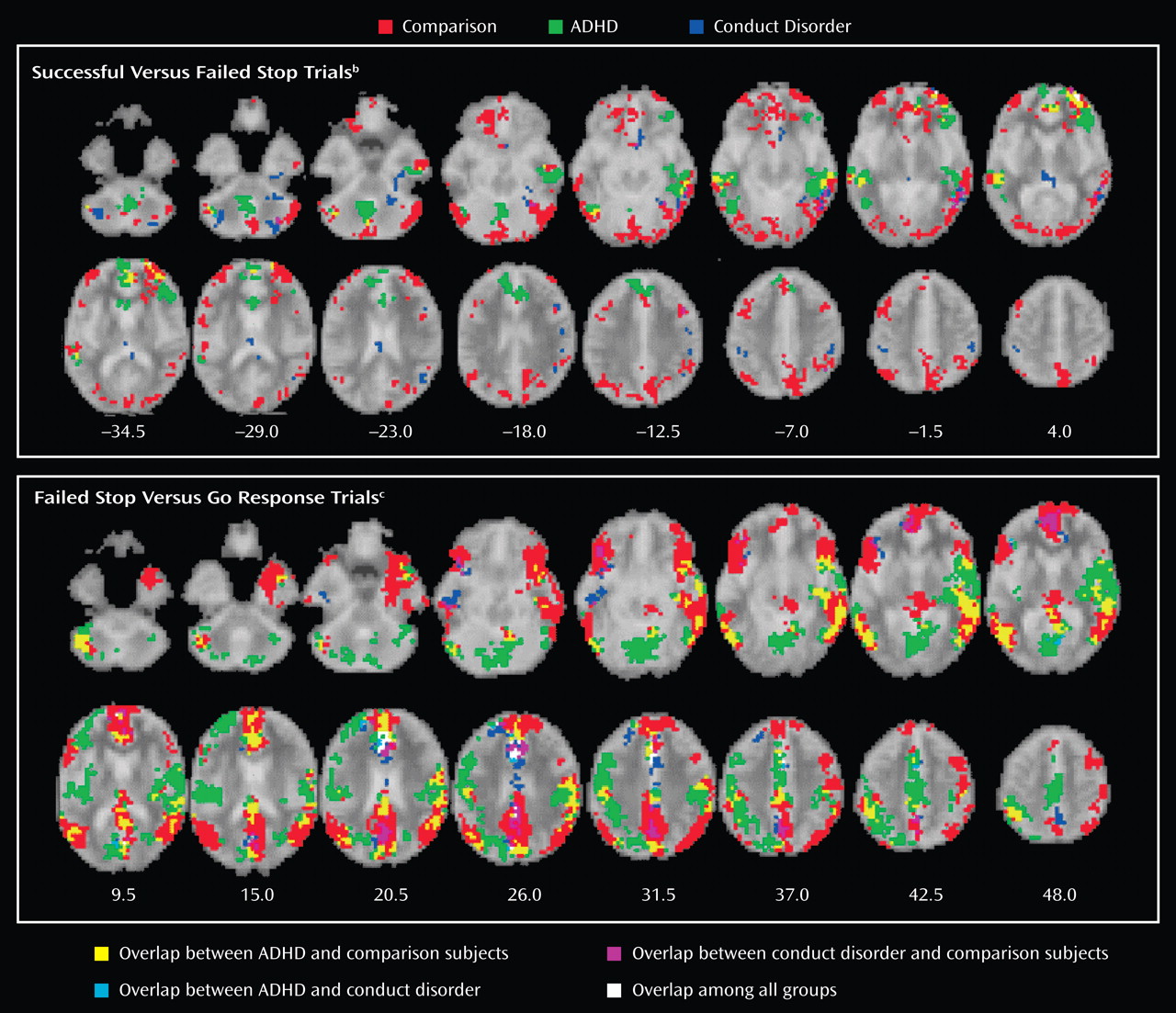
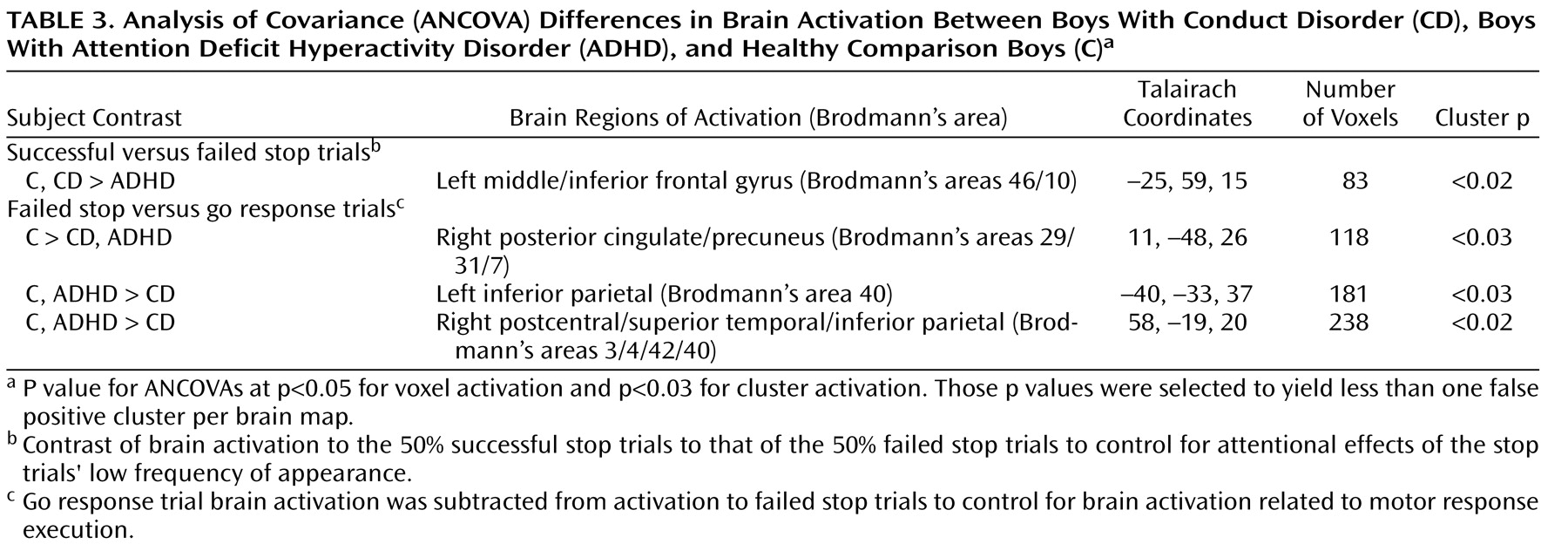
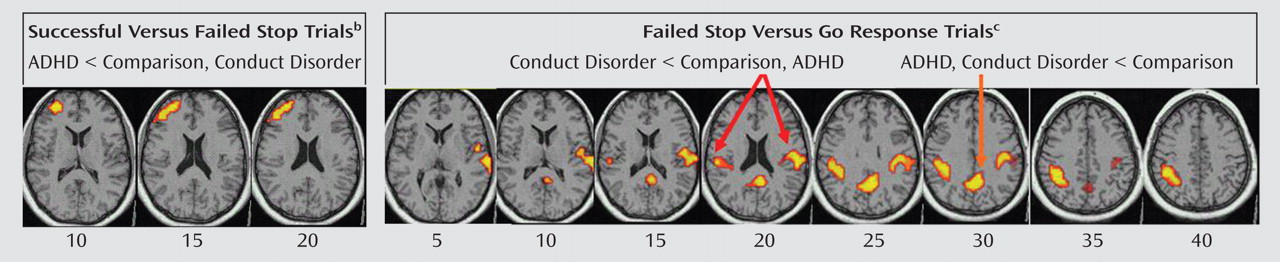
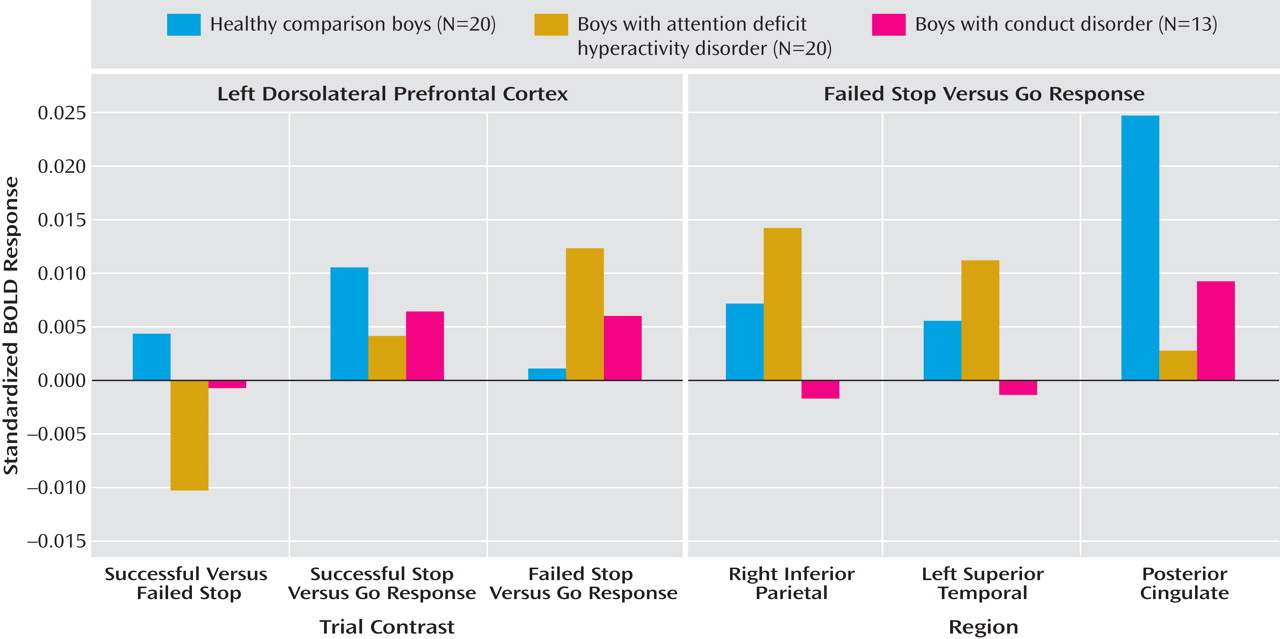
Children with noncomorbid forms of conduct disorder and ADHD show both similarities and differences in their brain activation abnormalities in relation to comparison subjects when performing a motor response inhibition task. During successful inhibition, when contrasted with failed inhibition, ADHD patients showed disorder-specific underactivation in relation to both comparison subjects and conduct disorder patients in the left dorsolateral/inferior prefrontal cortex that correlated with the speed of the inhibitory process in the comparison subjects. During failed inhibition, when contrasted with the go response, both patient groups shared reduced activation in relation to comparison subjects in the posterior cingulate gyrus; the conduct disorder group, in addition, showed disorder-specific temporal-parietal activation abnormalities compared to the other two groups.
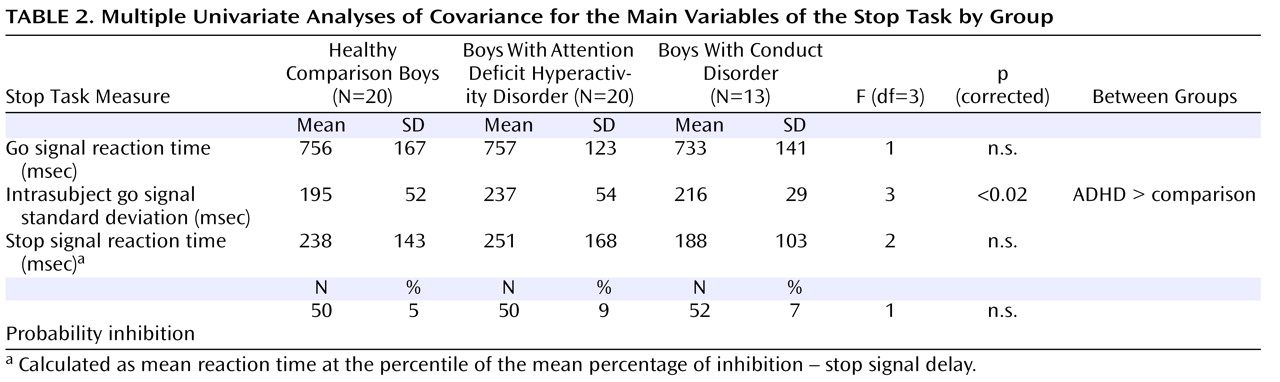
For successful versus failed inhibition trials, conduct disorder patients showed no brain activation differences in relation to comparison subjects, whereas ADHD patients showed disorder-specific differences in the left dorsolateral prefrontal cortex. The lack of brain activation differences in patients with conduct disorder could be the neural correlate for the behavioral findings that conduct disorder patients are more similar to comparison subjects than ADHD in their inhibitory capacity
(14) . In this study, although it did not reach significance, stop signal reaction time was slower in patients with ADHD than in patients with conduct disorder, and this may have reached significance with larger subject numbers.
The dorsolateral and inferior prefrontal cortices are typically underactivated in patients with ADHD during inhibition tasks
(2,
6,
31) . In a previous study that used the same task and included 10 ADHD patients who were also included in this study but that included other medication-naive ADHD children who scored higher on disruptive disorders than this group and included comorbidity with conduct disorder
(7), we observed a right-hemispheric and more inferior activation focus during successful inhibition
(7) . Other studies, however, have also found left dorsolateral prefrontal cortex underactivation in patients with ADHD during the go/no-go and stop tasks
(4,
5,
8), and this area is a key frontal abnormality in ADHD patients in a meta-analysis of fMRI studies
(32) . The differences in laterality and the exact location of prefrontal abnormalities in ADHD during inhibition tasks may be due to differences in the behavioral characteristics of the group; patients who are carefully selected to have low disruptive behaviors may differ in their brain abnormalities from patients with the more typical disruptive or comorbid presentation of ADHD
(1) .
During failed inhibition compared to go trials, conduct disorder patients showed reduced activation in relation to comparison subjects in the posterior cingulate gyrus, precuneus, and bilateral parietal regions that in the left hemisphere reached into the superior temporal cortex, precentral gyrus, and insula. The posterior cingulate underactivation in relation to comparison subjects was shared with ADHD patients, whereas the temporal-parietal abnormalities were disorder-specific. Parietal-temporal and cingulate brain regions are typically activated during inhibition tasks, in particular during inhibition failures
(25,
26,
33) . The participants obtain implicit feedback about their inhibition failures because the stop signal appears after they make their executive response. The activation in the posterior cingulate, precuneus, and parietal-temporal regions after errors has been suggested to reflect error detection and subsequently enhanced functions of arousal, attention allocation, and performance monitoring that are necessary to avoid future mistakes
(7,
25,
26) . The role of temporal-parietal brain regions in attention allocation is supported by findings of activation during oddball tasks
(34) . The cingulate and temporal-parietal underactivation in patients with conduct disorder may thus suggest a problem with the recruitment of attention allocation networks rather than inhibition networks. The disorder-specificity of temporal-parietal underactivation in patients with conduct disorder could suggest that performance monitoring networks are more dysfunctional in conduct disorder than in ADHD. This could mean that children with conduct disorder care less about their mistakes than both ADHD and comparison subjects, which would be in line with evidence that conduct disorder/oppositional defiance disorder children are undermotivated and respond less to negative feedback than comparison subjects
(35,
36) . The interpretation of the cingular-parietal-temporal underactivations as a reduced recruitment of attention allocation networks are also in line with EEG studies that found abnormal temporal-parietal activation in children with conduct disorder in relation to comparison subjects during sustained
(16) and selective attention tasks
(17) . The right temporal dysfunction is interesting with respect to the documented relationship between the temporal lobes and aggression
(37) and structural findings of smaller temporal volumes in patients with conduct disorder
(19) . Temporal lobe activation was furthermore reduced in conduct disorder patients compared to ADHD patients during successful stop compared to go trials. These findings demonstrate for the first time to our knowledge in fMRI that temporal and parietal brain regions are dysfunctional in conduct disorder and, furthermore, that these dysfunctions are specific to children with conduct disorder in the context of inhibitory control.
The finding of posterior cingulate underfunction in ADHD is in line with several previous findings during the same stop task in a more comorbid sample
(7), during rewarded sustained attention (unpublished study by Rubia et al.), and during a motor delay task
(2,
6) . Furthermore, in two of these studies, the cingulate dysfunctions were correlated with ADHD symptom scores (
7, unpublished study by Rubia et al.). The posterior cingulate is connected to the limbic system and visuomotor pathways and is relevant for the dynamic allocation of visual-spatial attention, in particular to visually or motivationally salient events
(38), which would be the perception of errors in this study. Dysfunction in this brain region thus seems to be a consistent and yet relatively neglected finding in ADHD that could be the neural substrate for age-inappropriate attention allocation.
The present findings of functional brain abnormalities in ADHD adolescents with low disruptive behaviors in lateral prefrontal and posterior cingulate regions replicate and extend our previous abnormality findings in more typical patients with ADHD during response inhibition. This study is the first to our knowledge, however, to demonstrate that dysfunction in the posterior cingulate is shared with patients with conduct disorder. A dysfunction in cingulate-mediated attention allocation and performance monitoring networks may thus be common to both disorders and could explain shared problems with visual-spatial attention. Both patient groups also shared underactivation during the go compared to the stop contrast in the anterior cingulate, basal ganglia, thalamus, and premotor regions, suggesting shared abnormalities in motor networks in relation to frequent versus no response execution.
A limitation of the study design is that we did not include a rest condition. The contrast of the task conditions with a resting or lower-level baseline condition could have provided additional information that might have further clarified the main group effect findings
(39) . ADHD children, however, are known to differ from comparison subjects in their brain activation during rest
(40,
41), and a resting condition may therefore not be more disambiguating than a go condition.
To our knowledge, this is the first neuroimaging study on carefully selected relatively “pure” patient groups who differed from each other in conduct and ADHD problems; previous studies investigated patient groups with a greater overlap between conduct disorder and ADHD symptoms or comorbidity
(16,
18,
19) .
In conclusion, this study shows similarities as well as qualitative dissociations of brain abnormalities in patients with ADHD and conduct disorder during inhibitory control. Both groups showed posterior cingulate abnormalities in response to inhibition failures, presumably reflecting a suboptimal performance monitoring network. However, temporal-parietal abnormalities were specific to patients with conduct disorder when compared to those with ADHD, suggesting a qualitative difference. Furthermore, only ADHD patients showed lateral prefrontal abnormalities during inhibition, suggesting that this deficit is specific to ADHD.
This study is a first step toward delineating the underlying neurobiological differences between the two diagnostic disorders in relation to a commonly affected and behaviorally not distinguishable process, i.e., inhibitory control. The nosological implication is to strengthen the independent recognition of both. A thorough delineation of the differences and commonalities of the underlying pathophysiology of these two behaviorally overlapping disorders will provide a more accurate and objective differentiation of the disorders that will ultimately help to develop disorder-specific treatment.