Sleep disturbance constitutes one of the most common difficulties facing older adults, with nearly 60% of the community-dwelling elderly reporting sleep problems at least a few nights per week
(1,
2) . Unfortunately, sleep difficulties are often considered to be a part of normal aging
(3), and assessment of sleep quality is frequently neglected during routine clinical evaluation
(4,
5) . This omission is particularly striking given evidence that sleep disturbance is associated with declines in health functioning, and with increases in all-cause mortality in older adults
(6,
7) . Moreover, sleep disturbance is often related to depressive disorders in late life
(8), which carry further considerable risks for morbidity and mortality.
Insomnia is the most frequent sleep disturbance in depressed patients, and such sleep impairment is viewed as a symptomatic dimension of current depression. Sleep disturbance often lingers and its persistence can represent a residual phase of a major mood disorder. Alternatively, emergence of disturbed sleep may serve as a precursor or prodrome of depression that occurs later in life. Indeed, as part of the Epidemiologic Catchment Area study, Ford and Kamerow
(9) found that persistent insomnia was associated with the incidence of new major depression. These findings identified the need for research to determine if early recognition and treatment of sleep disturbance, a modifiable risk factor
(10), might forestall or prevent incident depression.
Less is known about the prospective association between sleep disturbance and depression recurrence, as opposed to incident new major depression. Indeed, no study to our knowledge has examined this question in community-dwelling older adults. For example, whereas sleep disturbance has been shown to predict depression relapse and recurrence in late life
(11 –
16), these data are limited to convenience samples of specialty clinics. Other studies have examined factors associated with persistence of depression, as opposed to recurrence, in community-based older adults
(17 –
20), but none have tested the contribution of sleep disturbance. Moreover, in testing the association between sleep disturbance and late-life depression (i.e., including both first incidence and recurrence), no prior work took into account the role of other depressive symptoms and prior depression
(21 –
29) . For example, in a meta-analysis of 20 prospective studies
(8), only four studies used multivariate approaches to identify sleep disturbance as a predictor of depression onset among elderly community subjects
(21,
23,
25,
27), and among these mixed meta-analytic findings it is not known whether prior depression or severity of other depressive symptoms contributes to the risk profile of sleep disturbance and/or whether sleep disturbance is an independent risk factor for depression recurrence among those with a prior history of depression.
In this 2-year longitudinal study of community-dwelling older adults, we tested three hypotheses: 1) depression risk would be higher among those with a prior history of depression, 2) among those with prior depression, sleep disturbance would predict depression relapse or recurrence, and 3) sleep disturbance would act as a risk factor for depression recurrence independent of other depressive symptoms. We tested these hypotheses with repeated diagnostic ascertainment of incident major and nonmajor depressive disorders as diagnosed by the Structured Clinical Interview for the DSM-IV (SCID)
(30) in a sample of community-dwelling older adults who either had a prior lifetime history of depression or were never mentally ill, taking into account sociodemographic characteristics, chronic medical disease, antidepressant medication use, and characteristics of prior depressive episodes such as duration of last depressive episode and number of prior major depressive episodes.
Method
Subjects and Procedure
The current article reports on the Depression Substudy of the Veterans Affairs Cooperatives Trial #403, Shingles Prevention Study
(31) . The institutional review boards of the University of Colorado, the University of California at San Diego, and the University of California at Los Angeles approved all procedures.
As detailed elsewhere
(32), the Depression Substudy screened 2,858 subjects ages 60 years and older from three urban communities—Denver, Los Angeles, and San Diego—to identify persons who had evidence of a current or past history of a depression for further interview (N=220). In addition, a sample of age- and gender-matched screened participants who did not meet depression screening criteria were selected as comparison subjects (i.e., never been mentally ill, N=211).
At baseline, all initially selected subjects underwent a psychiatric diagnostic interview with administration of the SCID
(30), with characterization of the most recent three episodes (e.g., major versus nonmajor depression, medication used, date of remission). Depression severity was assessed through self-completion of the Beck Depression Inventory (BDI)
(33) . Because this study evaluated sleep disturbance as a risk factor and depressive symptom severity was used as a baseline covariate in the analyses, scores on the BDI were generated with removal of the single sleep item from this scale (i.e., BDI-S). The Pittsburgh Sleep Quality Index (PSQI) was used to assess perceived sleep disturbance—evaluating components such as subjective sleep quality, sleep latency, and use of sleep medications—during the past month and to screen for clinical sleep disturbance, defined as a PSQI score greater than five (sensitivity >90% and specificity >85%)
(34,
35) . We used sleep disturbance as a binary variable throughout the study (absent if PSQI ≤5 versus present if PSQI >5). The Chronic Disease Score (CDS) was determined as a standardized measure of chronic medical burden based on self-reported prescription medication use
(36) . The 36-item Short Form Health Survey (SF-36)
(37) assessed physical functioning, bodily pain, and social functioning as these factors have been related to depression outcome in older adults
(18,
19) . Similarly, given the associations between anxiety symptoms, sleep disturbance, and depression, a composite score of four items (agitation, psychic, or somatic anxiety, and hypochondriasis) was derived from the Hamilton Depression Rating Scale as previously described in order to assess anxiety symptoms
(16,
38) .
Of the 220 older adults who screened positive for depression or prior depression, 72 were excluded because of current depressive disorders and three because of breathing-related sleep disorder. The remaining 145 subjects reported a prior depression history (either major or nonmajor depressive disorder, N=111 and N=34, respectively); none fulfilled diagnostic criteria for a current depressive disorder or any other current DSM-IV disorder. Of the 211 subjects who did not screen positive for depression or prior depression, four were excluded because of past history of an axis I disorder and one because of a breathing-related sleep disorder. The final sample at baseline (N=351), therefore, consisted of the following two groups: prior depression history (N=145) and control (never mentally ill, N=206).
Follow-up assessments were conducted at 6 weeks, 1 year, and 2 years from baseline. At each follow-up visit, all subjects underwent the SCID interview with ascertainment of major- and nonmajor depressive disorders. Of 351 subjects included at baseline, 349 (99.4%), 335 (95.4%), and 329 (93.7%) were assessed respectively at 6 weeks, 1 year, and 2 years.
Statistical Analysis
Stata Version 9.2 (StataCorp, College Station, Tex.) was employed for all statistical analyses and the significance level was set at p≤0.05. Participant characteristics at baseline were compared between the groups by two-tailed chi-square or t tests. The cumulative incidence of depressive disorders as ascertained using the SCID was estimated with the appropriate 95% confidence interval (CI) at three follow-up visits for each group.
Given the longitudinal and repeated nature of measurement, logistic regression using a random effects model was adopted
(39) . As all the independent variables were either a priori potential risk factors for depression (group status [prior depression versus control], sleep disturbance [PSQI], baseline depressive symptoms [BDI-S], and medical burden [CDS]) or sociodemographic characteristics (age, gender, marital status, and education), they were forced into the multivariable model. As the absolute majority of the sample was Euro-American, ethnicity was not considered in further analyses. Additional exploratory analyses included measures of physical functioning, bodily pain, social functioning, and anxiety symptoms to test a more comprehensive multivariable model. Effects of baseline variables on severity of depressive symptoms (i.e., BDI total score) during follow-up were also evaluated, in an effort to understand more subtle changes of depression that do not fulfill the criteria of the SCID depressive disorders. Finally, to assess a dose-response effect of prior depression on subsequent SCID depressive disorders, logistic regression was conducted in which group status was recategorized into “no prior depression,” “prior nonmajor depression,” and “prior major depression.”
Further analyses were conducted within the prior depression group to identify whether sleep disturbance predicted depression relapse or recurrence. Survival analysis was adopted for this step, and hence time to depression relapse or recurrence was calculated based on information in the follow-up SCID data. The prior depression group was divided into two subgroups according to the presence or absence of sleep disturbance at baseline, and survival analysis was implemented comparing time to first relapse or recurrence of depression in each subgroup. Analyses included both a simple subgroup comparison (Kaplan-Meier estimate) and a multivariable comparison with covariates (Cox regression). The covariates included baseline depressive symptoms, baseline antidepressant use, baseline medical burden, time since last depressive episode (more than 5 years versus 5 years or less), duration of last depressive episode (6 months or less versus more than 6 months), number of prior major depressive episodes (none or single versus two or more), and sociodemographic variables. Sleep medication use was not included as a covariate because it was a component of the composite PSQI score, which defined sleep disturbance in our study. However, additional exploratory analyses were performed to examine whether sleep medication use, as a separate variable, predicted temporal change of the PSQI score (recalculated without the sleep medication item for this purpose), as well as depression recurrence, respectively using random-effects linear regression and Cox regression. Subsequently, a number needed to treat (NNT) value was estimated to determine the clinical impact of treating sleep disturbance to prevent depression recurrence. NNT was calculated in a multivariable context with control for covariates with adjusted hazard ratio obtained from Cox regression, following a published guideline
(40) . The calculation was based on the following equation:
NNT=1/{[S c (t)] HR –S c (t)}
where S c (t) is the depression free survival probability in an untreated group at 2 years: t . Finally, as above, exploratory analyses included measures of physical functioning, bodily pain, social functioning, and anxiety symptoms in the model.
Results
Characteristics of Study Participants
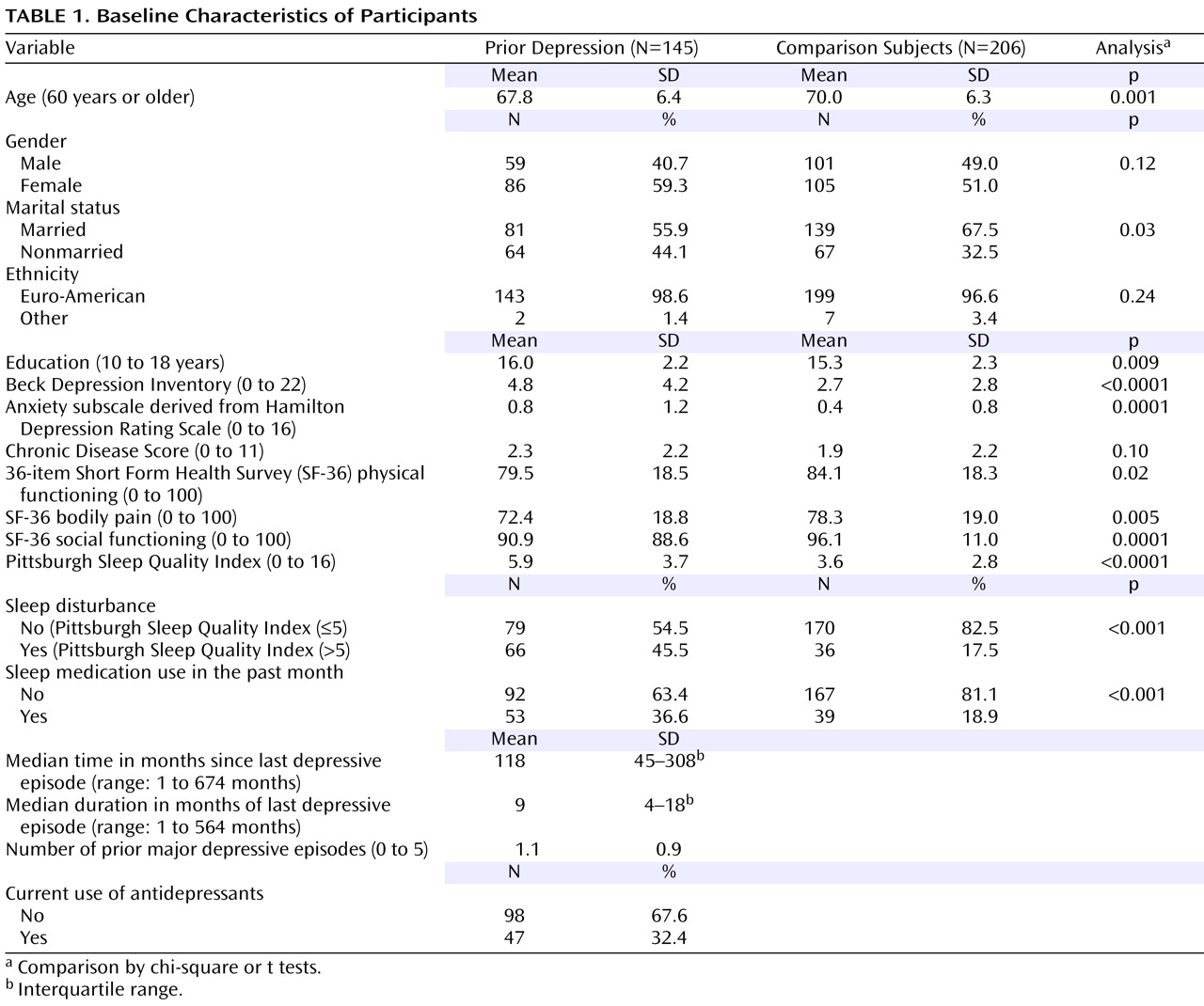
Table 1 describes the baseline characteristics of the study participants. There was no significant difference between the two groups regarding gender, ethnicity, and chronic medical burden. However, several characteristics significantly differed. The prior depression group was younger, more educated, and less likely to be married and presented higher severity of depressive symptoms and worse sleep quality. Within the prior depression group, the level of depressive symptoms was quite low (mean BDI score at a level considered “no or minimal symptoms”), while the level of sleep problems was relatively high, with 46% of the group classified as cases of clinical sleep disturbance as defined by PSQI>5. It is notable, however, that nearly 18% of the control subjects were also classified as having clinical sleep disturbance. Similarly, according to the information gathered using the PSQI, 19% of the control subjects used sleep medications (prescribed or over-the-counter) at least once during the past month, compared to 37% of the prior depression group. As to the characteristics of prior depressive episodes, the median time since last depressive episode was nearly 10 years, indicating a period of sustained full remission for most in the prior depression group. In addition, the median duration of last depressive episode was 9 months, with, on average, only one lifetime episode of a major depressive disorder. Nearly one-third of the prior depression group was using antidepressant medications at baseline.
During the follow-up, only one control subject developed a depressive disorder. In contrast, among the prior depression group, the cumulative incidence of depressive disorders was 4.9% (95% CI=2.0% to 9.8%) at 6 weeks; 12.6% (95% CI=7.5% to 19.4%) at 1 year; and 16.9% (95% CI=11.0% to 24.3%) at 2 years. All depression cases in the latter group developed after at least 10 months of full remission and were considered “recurrences” since 6 months of full remission is required for this definition
(40) ; the term “relapse” is omitted hereafter. In addition, depression recurrence during the follow-up was over 8 months after the baseline assessment.
Baseline Predictors of Depression Occurrence
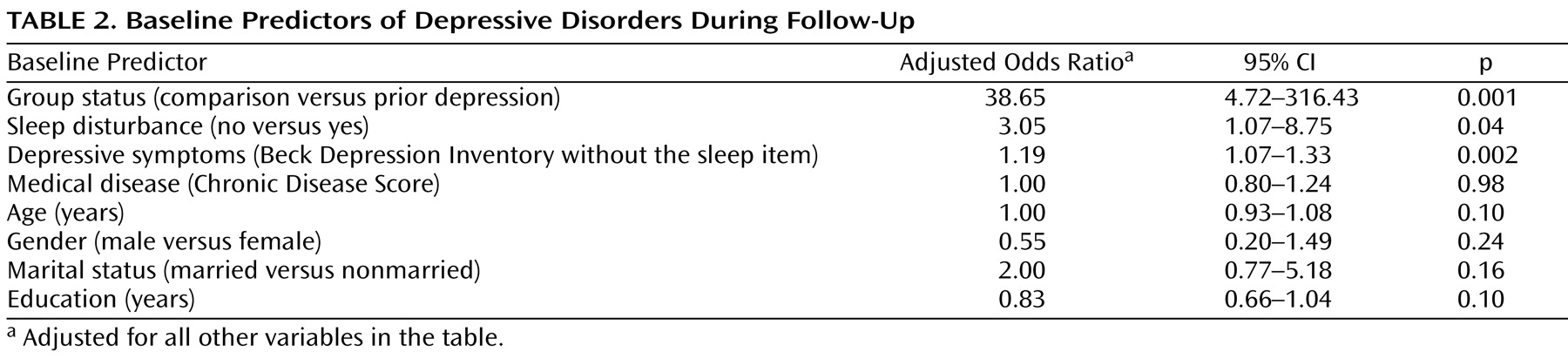
Consistent with our hypothesis, prior depression prospectively identified depression outcome over the 2-year follow-up. Depression occurrence was independently associated with group status (prior depression versus control; adjusted odds ratio=38.65, 95% CI=4.72 to 316.43, p=0.001), and also with sleep disturbance (odds ratio=3.05, CI=1.07 to 8.75, p=0.04) and other depressive symptoms (odds ratio=1.19, CI=1.07–1.33, p=0.002) (
Table 2 ). Exploratory analyses included additional covariates (i.e., anxiety symptoms, physical functioning, bodily pain, and social functioning) and found that depression occurrence remained associated with group status (odds ratio=39.52, CI=4.09 to 381.51, p=0.001), but neither with sleep disturbance (odds ratio=2.48, CI=0.81 to 7.57, p=0.11) nor other depressive symptoms (odds ratio=1.06, CI=0.93 to 1.22, p=0.38).
Further analyses tested the association between the main independent variables (prior depression and sleep disturbance) and depressive symptom severity (i.e., BDI scores) as the dependent variable and also yielded significant results. In regard to the association between sleep disturbance and depression outcome, this relationship appeared to be operating primarily in the prior depression group. As noted above, a substantial number of controls were classified as having clinical sleep disturbance (18%), yet only one case of depression was found in this group. There was, however, a significant association between baseline sleep disturbance and depressive symptom severity during follow-up in the control group.
Additional analyses focused on the role of prior depression on depression outcome and examined whether prior depression history showed a gradient effect. Prior major depression, prior nonmajor depression, and the control group were used as group status predictors. Adjusting for sleep disturbance, other depressive symptoms, chronic medical burden, and sociodemographic variables, prior major depression was found to be a stronger predictor of subsequent depression than prior nonmajor depression, with respective adjusted odds ratios of 49.37 (95% CI=5.92–411.67, p<0.001) and 13.73 (CI=1.19–158.56, p=0.04).
Baseline Predictors of Depression Recurrence
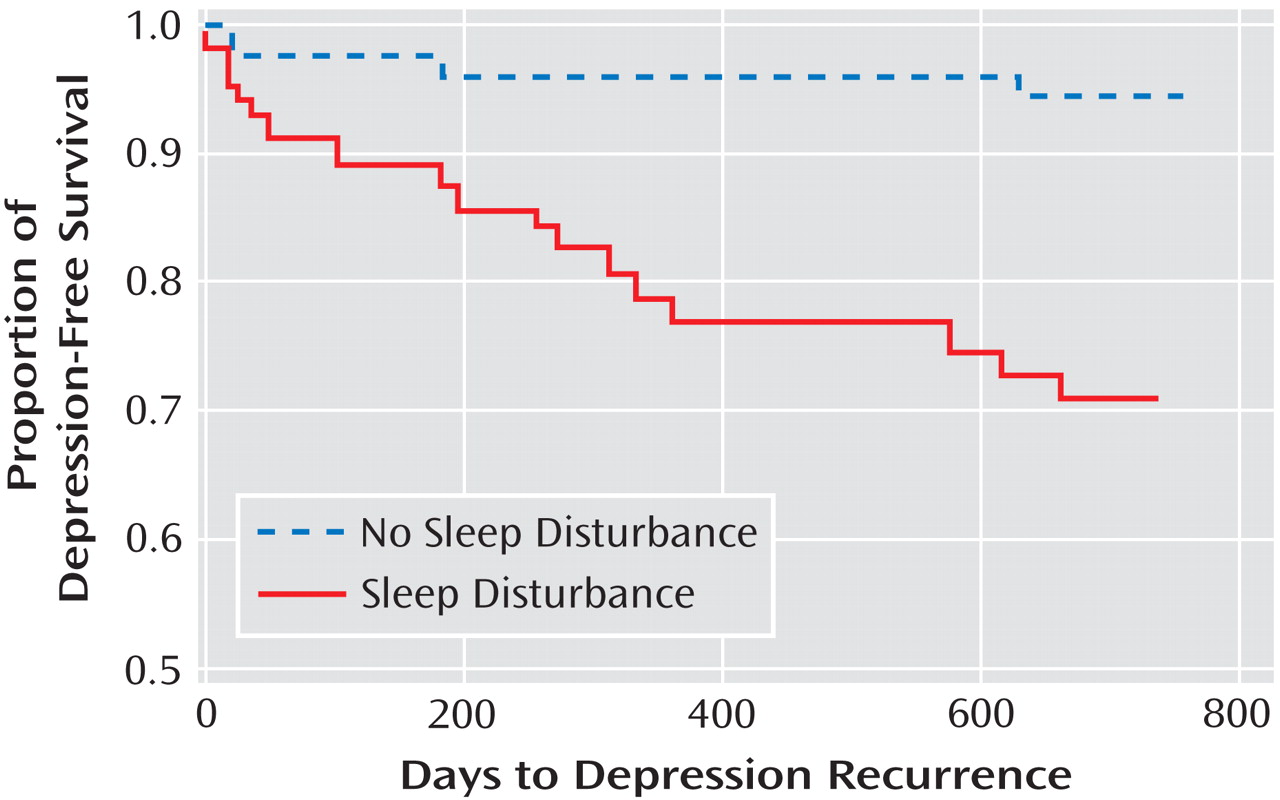
Given that the association between sleep disturbance and depression was primarily found in the prior depression group, we tested the second hypothesis to determine whether sleep disturbance is a risk factor for depression recurrence among those with prior depression. Hence, these analyses focused solely on community-dwelling older adults with prior depression, stratified by the presence or absence of sleep disturbance at baseline. Using survival analysis for time to depression recurrence, sleep disturbance was a powerful predictor of depression recurrence in the prior depression group (Mantel-Cox χ
2 =13.36, df=1, p<0.001). As shown in
Figure 1, nearly all cases of depression occurred in those older adults who were classified as having sleep disturbance at baseline.
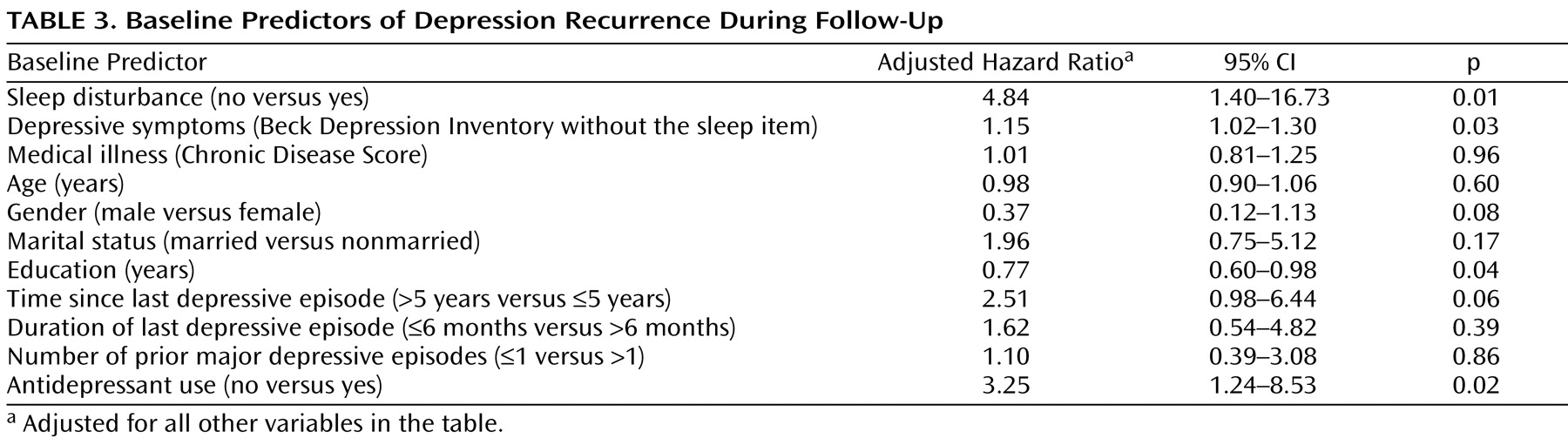
Further analyses were conducted using a Cox regression model to control for baseline covariates (
Table 3 ). The presence of sleep disturbance at baseline remained significantly associated with depression recurrence (adjusted hazard ratio=4.84, 95% CI=1.40–16.73, p=0.01). Of the covariates, the following were statistically significant predictors of recurrence: antidepressant medication use (odds ratio=3.25, CI=1.23–8.53, p=0.02), other depressive symptoms (odds ratio=1.15, CI=1.02–1.30, p=0.03), and education (odds ratio=0.77, CI=0.60–0.98, p=0.04) (
Table 3 ). In separate exploratory analyses, sleep medication use at baseline predicted neither the temporal change of sleep quality (adjusted regression coefficient=–0.003, CI=–0.01 to 0.004, p=0.41) nor the recurrence of depression (adjusted hazard ratio=1.07, CI=0.41 to 2.82, p=0.89).
The estimated NNT value was 5 (95% CI=4 to 14) assuming 100% efficacy of sleep disturbance treatment; with 50% efficacy of sleep disturbance treatment, this estimate was 10 (CI=8 to 29). Finally, exploratory analyses included additional covariates (i.e., anxiety symptoms, physical functioning, bodily pain, and social functioning) and found that depression recurrence remained predicted by sleep disturbance (odds ratio=6.16, CI=1.48–25.69, p=0.01), antidepressant medication use (odds ratio=3.95, CI=1.42–11.01, p=0.009), and education (odds ratios=0.76, CI=0.59–0.98, p=0.03), but not by other depressive symptoms (odds ratios=1.03, CI=0.89–1.18, p=0.73).
Discussion
This 2-year longitudinal study of community-dwelling older adults confirmed our study hypotheses. A history of prior depression predicted depression recurrence during the follow-up period. Second, among those with prior depression, the presence of sleep disturbance was associated with a substantially higher risk of depression recurrence. Sleep disturbance functioned primarily as a depression risk factor in those who have had a prior depression; whereas a substantial proportion of never-depressed control subjects showed evidence of sleep disturbance, only one control subject developed a new late-onset depression at follow-up. Finally, among those who had a prior depression, we provide novel data that sleep disturbance predicted depression recurrence, independent of other depressive symptoms, sociodemographic characteristics, chronic medical burden, and antidepressant medication use. Among community-dwelling older adults with a prior depression, these prospective cohort data are the first, to our knowledge, to demonstrate that sleep disturbance is a risk factor for depression recurrence as ascertained by diagnostic interview, independent of baseline depressive symptoms.
The results regarding sleep disturbance as a risk factor for depression recurrence substantially extend prior studies
(8) . Whereas sleep disturbance can be either a residual or prodromal symptom of depression, this study suggests that sleep disturbance likely functions as an independent risk factor for depression recurrence. Among those with a prior depression history, median duration of remission was nearly 10 years. Moreover, although almost half of this group showed evidence of clinical sleep disturbance, they presented only minimal depressive symptoms, which were further covaried in the analyses. Finally, during the 2-year follow-up, recurrence of depression in most cases occurred months—possibly years—after the assessment of sleep disturbance; median time to depression recurrence was more than 8 months. Hence, our study is unique in its simultaneous evaluation of the contribution of sleep disturbance, prior depression, and depressive symptoms to risk of depression recurrence in community-dwelling older adults
(23) . In convenience samples treated in psychiatric settings, sleep disturbance, as defined by the PSQI, was similarly related to depression recurrence
(11,
16), with further data showing that residual depressive symptoms were risk factors for relapse and recurrence of depression during the maintenance treatment
(14,
16) . Furthermore, our data suggest that the risk posed by sleep disturbance is greater—maybe specific—for depression recurrence than for depression occurrence, consistent with previous studies, which showed that insomnia present only at baseline was not prospectively associated with incident depression in the general population
(9) or in community-residing older adults
(27) .
Lifetime history of either major or nonmajor depression predicted depression outcome at follow-up in a gradient fashion; prior major depression was a stronger predictor of depression than prior nonmajor depression. Several studies have reported on the importance of nonmajor or subthreshold depressions in elderly patients
(41,
42) . Likewise, prior depression history has been consistently reported to be a risk factor for late-life depression
(43,
44) . However, no previous study has assessed a dose-response effect of major compared to nonmajor depression history on depression recurrence.
Several limitations influence interpretation of these findings. First, because the present study was a substudy of the SPS herpes zoster vaccine trial, the study sample had low levels of medical morbidity, and hence, it was not entirely representative of the community-dwelling elderly population. Second, as the sample was primarily white, the findings may not be generalizable to ethnic minorities. Third, although our follow-up duration was comparable to previous older adult studies of depression risk
(24,
27), a longer follow-up period may be required, especially considering that there were few cases of depression in the control group. Fourth, it was not possible to encompass all possible risk factors for depression in this single study, for example, bereavement was not assessed
(8) . Fifth, the assessment of sleep quality relied on self-report, whereas polysomnography is arguably the objective standard to assess insomnia. However, the PSQI shows a high sensitivity (98.7%) and specificity (84.4%) in identifying insomnia
(35) . In addition, assessment of sleep problems in clinical practice primarily relies on patients’ subjective evaluation. Similarly, the assessment of prior depressive episodes depended on the recollection of participants through the SCID interview, although this method remains the gold standard for ascertainment of depression and was reported to be reliable in assessing depression history
(45) .
Several mechanisms have been proposed to explain how sleep disturbance leads to depression. For example, sleep problems lead to poor quality of life in older adults
(46), which in turn increases risk for late-life depression
(8) . However, we made an effort to control for this pathway by including health-related quality of life as a covariate in the multivariable models. Therefore, other mechanisms may be implicated, including activation of autonomic and inflammatory pathways by sleep disturbance, which are hypothesized to affect mood regulation and lead to depression
(47,
48) .
Our results suggest that a simple two-step strategy may be useful in identifying community-dwelling older adults at risk for depression relapse and recurrence. First, prior history of depression is a known nonmodifiable risk factor for late-life depression. Second, among those with a prior depression history, self-administration of the PSQI could determine the presence of sleep disturbance, which is a potentially modifiable risk factor for a recurrent depressive episode. Given that over one-third of those with prior depression history were using antidepressant medications, and such use was not protective in ameliorating the risk of sleep disturbance on depression recurrence, we suggest that interventions that specifically target insomnia have the potential to prevent depression relapse and recurrence and may be more effective than antidepressant medications for this purpose. Of the various sleep interventions available, it should be noted that whereas sedative hypnotic medications are routinely used to treat insomnia symptoms, a recent meta-analysis indicates that these medications in older adults carry substantial risks for falls and cognitive impairment that may outweigh their benefit in producing small improvements in sleep quality
(49) . In line with this notion, sleep medication use contributed neither to the temporal change of overall sleep quality nor the risk of depression recurrence in the prior depression group, with the caveat that determination of sleep medication use relied on self-report, included both prescription and over-the-counter medications, and did not assess whether the frequency or dosage of such use met clinical treatment adherence guidelines. In contrast, nonpharmacological behavioral interventions (e.g., cognitive-behavioral therapy) have been found to be effective in the treatment of insomnia in older adults without the risk profile of sedative-hypnotic medications
(10,
49 –
51) . To place our observations within a broader medical context, the number of patients needed to be treated with sleep disturbance interventions to prevent a recurrence of depression was 10, substantially lower compared to 21 for statins—a widely used medication to prevent another myocardial infarction
(52) .
In conclusion, sleep disturbance is substantially associated with the risk of depression recurrence in older adults and is suggested to be a priority target for clinical intervention to reduce depressive morbidity in older adults with a prior history of depression. Direct evidence on the effectiveness of sleep-related interventions in preventing late-life depression needs to be established in future studies.