Anhedonia—lack of reactivity to pleasurable stimuli—is a core symptom of major depressive disorder
(1,
2) . Relative to healthy comparison subjects, depressed individuals display reduced positive attentional biases
(3), weaker positive affect in response to pleasant stimuli
(4), and reduced reward responsiveness
(5) . Neuroimaging indicates that these deficits may reflect dysfunction in the basal ganglia, including the striatum (nucleus accumbens, caudate, putamen) and the globus pallidus
(6 –
11) . However, the functional significance of basal ganglia dysfunction in major depression remains poorly understood. Specifically, whether dysfunction is more closely associated with deficits in the anticipatory or the consummatory phase of reward processing is unclear.
Dissociating these phases is important for two reasons
(12) . First, they reflect different psychological states: anticipation is characterized by goal-directed behavior, whereas consummation involves pleasure experience
(13) . Second, they make separable contributions to goal-directed behavior
(14) . In nonhuman primates, unexpected rewards elicit phasic bursts in dopamine neurons projecting from the midbrain to the basal ganglia
(14) . However, the bursts eventually shift from the rewards to reward-predicting cues. Because the basal ganglia are critical for motor control
(15), this constitutes a mechanism by which reward-predicting cues can elicit motivated behavior. Given dopamine abnormalities in major depression
(16), depression may involve impairments in the anticipatory and/or consummatory components of this mechanism.
To explore this issue, a recent study
(17) used a monetary incentive delay task to investigate anticipatory versus consummatory phases of reward processing in 14 participants with major depression and 12 comparison subjects. Surprisingly, there were no group differences in basal ganglia responses to reward cues. Furthermore, although participants with major depression showed bilateral reductions in putamen responses to gains, no outcome-related differences emerged in the accumbens or the caudate, regions implicated in processing reward feedback
(18,
19), particularly when reward delivery is unpredictable
(20) . However, there were also no group differences in behavior. Thus, these null results may have reflected intact reward processing in that particular sample of patients with major depression and/or limited statistical power.
In this study, we used a similar task to probe anticipatory and consummatory phases of reward processing in a larger group of unmedicated depressed individuals (N=30) and healthy comparison subjects (N=31). To permit a balanced design, the task was modified such that 50% of reward and loss trials ended in monetary gains and penalties, respectively
(21) . Given the role of dopamine and the basal ganglia in reward anticipation
(22), we predicted that depressed individuals would show blunted responses to reward cues, particularly in the ventral striatum. However, based on prior findings
(17), and because gains would be delivered in only 50% of reward trials
(20), we hypothesized that participants with major depression might primarily show impaired striatal responses to rewarding outcomes. Finally, in light of recent work
(23), we predicted that greater anhedonic symptoms would be associated with smaller caudate volumes.
Method
Participants
Depressed individuals were recruited from a treatment study comparing the effectiveness of the dietary supplement
S -adenosyl-
l -methionine and escitalopram. Comparison subjects were recruited from the community. Participants with major depression had a DSM-IV diagnosis of major depressive disorder according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID;
24 ) and had a score ≥16 on the 21-item Hamilton Depression Rating Scale (HAM-D;
25 ). Exclusion criteria were any psychotropic medication in the past 2 weeks, fluoxetine in the past 6 weeks, or dopaminergic drugs or neuroleptics in the past 6 months; a current or past history of major depressive disorder with psychotic features; and presence of other axis I diagnoses (including lifetime substance dependence and any substance use disorder in the past year), with the exception of anxiety disorders. Comparison subjects reported no medical or neurological illness, no current or past psychopathology (according to the SCID), and no use of psychotropic medications. All participants were right-handed.
The final sample included 30 participants with major depression and 31 demographically matched comparison subjects (
Table 1 ). Participants with major depression were moderately depressed, as assessed by the Beck Depression Inventory–II (BDI;
26 ) (mean score=27.48 [SD=10.60]) and the 17-item HAM-D (mean score=17.97 [SD=4.19]). Eleven depressed participants had a current anxiety disorder, and three had subthreshold anxiety symptoms. In the major depression group, 11 participants (37%) had never received antidepressants and 16 (53%) reported prior antidepressant use; information about prior antidepressant treatment was unavailable for three individuals. Only three individuals reported resistance to a prior antidepressant. All participants provided written informed consent to a protocol approved by the Committee on the Use of Human Subjects in Research at Harvard University and the Partners Human Research Committee.
Monetary Incentive Delay Task
The task has been described previously
(21) . Trials began with a visual cue (1.5 seconds) indicating the potential outcome (reward: +$; loss: –$; no incentive: 0$). After a variable interstimulus interval (3–7.5 seconds), a red target square was briefly presented, to which subjects responded by pressing a button. After a second delay (4.4–8.9 seconds), visual feedback (1.5 seconds) indicated trial outcome (gain, penalty, no change). A variable interval (3–12 seconds) separated the trials. The task involved five blocks with 24 trials (eight per cue), yielding 40 and 20 trials for cue- and outcome-related analyses, respectively.
Participants were told that responding rapidly would maximize their chances of obtaining gains and avoiding penalties. However, gains and penalties were actually delivered in a predetermined pattern to allow a balanced design. For each block, half the reward trials yielded a monetary gain (range=$1.96–$2.34; mean=$2.15) and half ended with no-change feedback. Similarly, half the loss trials yielded a monetary penalty (range=$1.81–$2.19; mean=$2.00), and half resulted in no change. No-incentive trials always ended with no-change feedback. To maximize feedback believability, target duration was longer for trials scheduled to be successful (i.e., gains on reward trials) than for those scheduled to be unsuccessful (i.e., no-change feedback on reward trials). Furthermore, target durations were individually titrated on the basis of data collected on reaction time during a practice session (see the data supplement that accompanies the online edition of this article).
Procedure
Data collection occurred prior to start of treatment. After blocks 2 and 4, participants rated their affective response to cues and outcomes for valence (on a scale ranging from 1=most negative to 5=most positive) and arousal (from 1=low intensity to 5=high intensity). Participants were compensated $80 for their time and “earned” $20–$22 from the task.
Data Acquisition
Data were collected on a 1.5-T Symphony/Sonata scanner (Siemens Medical Systems, Iselin, N.J.) and consisted of a T
1 -weighted MPRAGE acquisition (repetition time=2730 msec; echo time=3.39 msec; field of view=256 mm; voxel dimensions=1×1×1.33 mm; 128 slices) and gradient echo T
2 *-weighted echoplanar images, which were acquired using an optimized pulse sequence
(21) (repetition time=2500 msec; echo time=35 msec; field of view=200 mm; voxel dimensions=3.125×3.125×3 mm; 35 interleaved slices).
Data Reduction and Statistical Analyses
Reaction time and affective ratings
After removal of outliers (responses exceeding three standard deviations from the mean), reaction time data were entered into a group-by-cue-by-block analysis of variance (ANOVA). For brevity, only effects involving group or cue are reported. Affective ratings were averaged across the two assessments and entered into group-by-cue or group-by-outcome ANOVAs.
Functional and structural MRI
Analyses were conducted using FreeSurfer and FreeSurfer Functional Analysis Stream (FS-FAST) (
27 ; http://surfer.nmr.mgh.harvard.edu). Preprocessing included slice-time and motion correction, removal of slow trends with a second-order polynomial, intensity normalization, and spatial smoothing (6 mm full width at half maximum); a temporal whitening filter was used to correct for autocorrelation in the noise. Data for four participants in the major depression group were lost because of excessive motion (>5 mm), leaving 26 individuals with major depression and 31 comparison subjects for fMRI analysis. Before group analyses were conducted, the data were resampled into the Montreal Neurological Institute MNI305 space (voxel size=2 mm
3 ).
Functional data were analyzed using the general linear model. The hemodynamic response was modeled as a gamma function and convolved with stimulus onsets; motion parameters were included as nuisance regressors. Between-group whole-brain random-effects comparisons were computed for reward anticipation (reward cue versus no-incentive cue) and reward outcome (gain versus no-change feedback on no-incentive trials) contrasts. Note that because of the double subtraction, clusters exceeding the statistical threshold show a significant group-by-condition interaction. Secondary analyses of loss-related contrasts are reported in the online data supplement. Because of a priori hypotheses about the basal ganglia, activation maps were thresholded using a peak voxel criterion of p<0.005 with a minimum cluster extent of 12 voxels; Monte Carlo simulations were performed to confirm that the primary findings held after correction for multiple comparisons (see the online data supplement). Findings emerging outside the basal ganglia should be considered preliminary. To assess whether findings in a priori regions were specific to rewards, follow-up group-by-condition ANOVAs were conducted on averaged beta weights (including for penalties) extracted from clusters showing group differences.
Structural MRI
Morphometric analyses used FreeSurfer’s automated parcellation approach
(27,
28 ; see Table S1 in the online data supplement) and focused on the basal ganglia. To account for differences in cranial size, volumes were divided by the intracranial volume and entered into a group-by-hemisphere-by-region (nucleus accumbens, caudate, putamen, and globus pallidus) ANOVA. Significant effects were followed up with post hoc t tests. For participants with major depression, Pearson correlations and hierarchical regressions (controlling for age and gender) were conducted to examine relationships between volumes and anhedonic symptoms or depression severity. As in prior work
(29), anhedonia was assessed by computing a BDI anhedonia subscore (loss of pleasure, interest, energy, and libido; reliability coefficient: a=0.85).
Results
Reaction Time
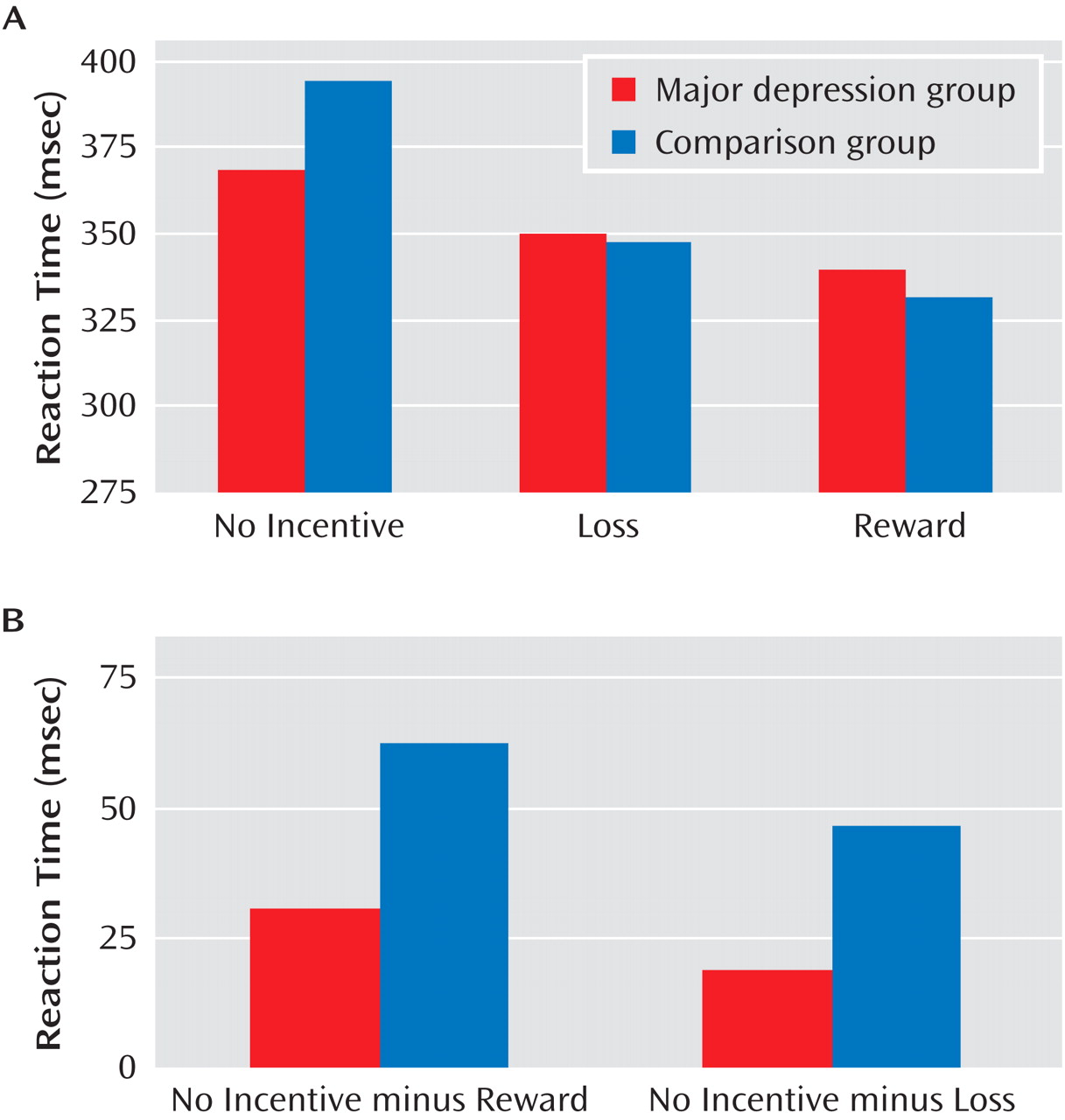
A main effect of cue emerged (F=30.15, df=2, 118, p<0.0001), reflecting motivated responding (shorter reaction time) on reward and loss trials versus no-incentive trials. The main effect of group was not significant, indicating that the comparison and major depression groups showed similar overall reaction time (mean=350.38 msec [SD=68.91] and mean=357.01 msec [SD=75.60], respectively; see the online data supplement). These effects were qualified by a significant group-by-cue interaction (F=3.98, df=2, 118, p<0.045). As evident from
Figure 1 A, the interaction reflected smaller reaction time differences on incentive versus no-incentive trials in the major depression group. Relative to comparison subjects, participants with major depression showed weaker reward-related reaction time modulation (reaction time in no-incentive trials minus reaction time in reward trials; t=–2.09, df=59, p<0.047), with a similar tendency for loss-related reaction time modulation (t=–1.97, df=59, p=0.053) (
Figure 1 B). However, no group differences in reaction time emerged for reward, loss, or no-incentive trials (p values >0.21). Moreover, both groups showed the shortest reaction time to reward cues, followed by loss and no-incentive cues (p values <0.002).
Mirroring the lack of group effect in reaction times collected during scanning, groups did not differ in target durations linked to successful or unsuccessful outcomes, which were selected on the basis of reaction time during practice (see the online data supplement). There were also no group differences in the percentage of reward trials ending in gains or of loss trials ending in penalties, or in total money earned (see Table S2 in the online data supplement). Thus, fMRI findings were not confounded by group differences in task difficulty.
Affective Ratings
Participants’ ratings data indicated that the cues and outcomes elicited the intended responses (see Figure S1 in the online data supplement). Critically, relative to the comparison group, the major depression group reported overall reduced positive affect in response to both cue (group: F=5.62, df=1, 58, p<0.021) and feedback (group: F=12.26, df=1, 59, p<0.001) stimuli, as well as reduced arousal in response to gains (p<0.045) but not to penalties or no-change feedback (p values >0.42; group-by-outcome interaction, F=3.20, df=2, 118, p<0.045).
Functional MRI Data
Reward anticipation (reward cue minus no-incentive cue)
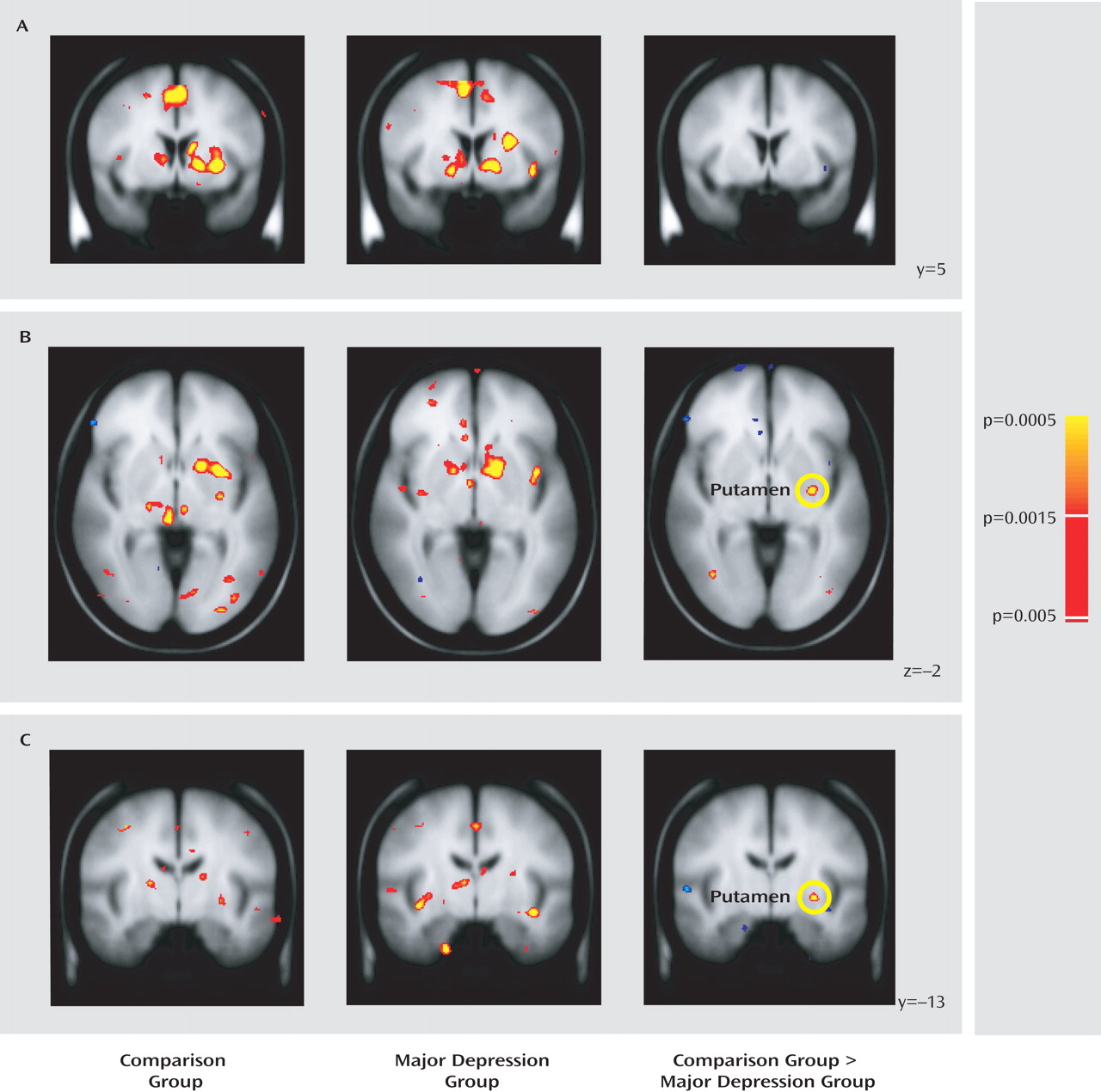
A complete list of regions showing group differences is provided in Table S3 of the online data supplement. Surprisingly, both groups showed robust basal ganglia responses to reward cues (
Figure 2 A). However, the major depression group showed relatively weaker activation in the left posterior putamen (
Figure 2 B and C).
Reward outcome (gain minus no-change feedback)
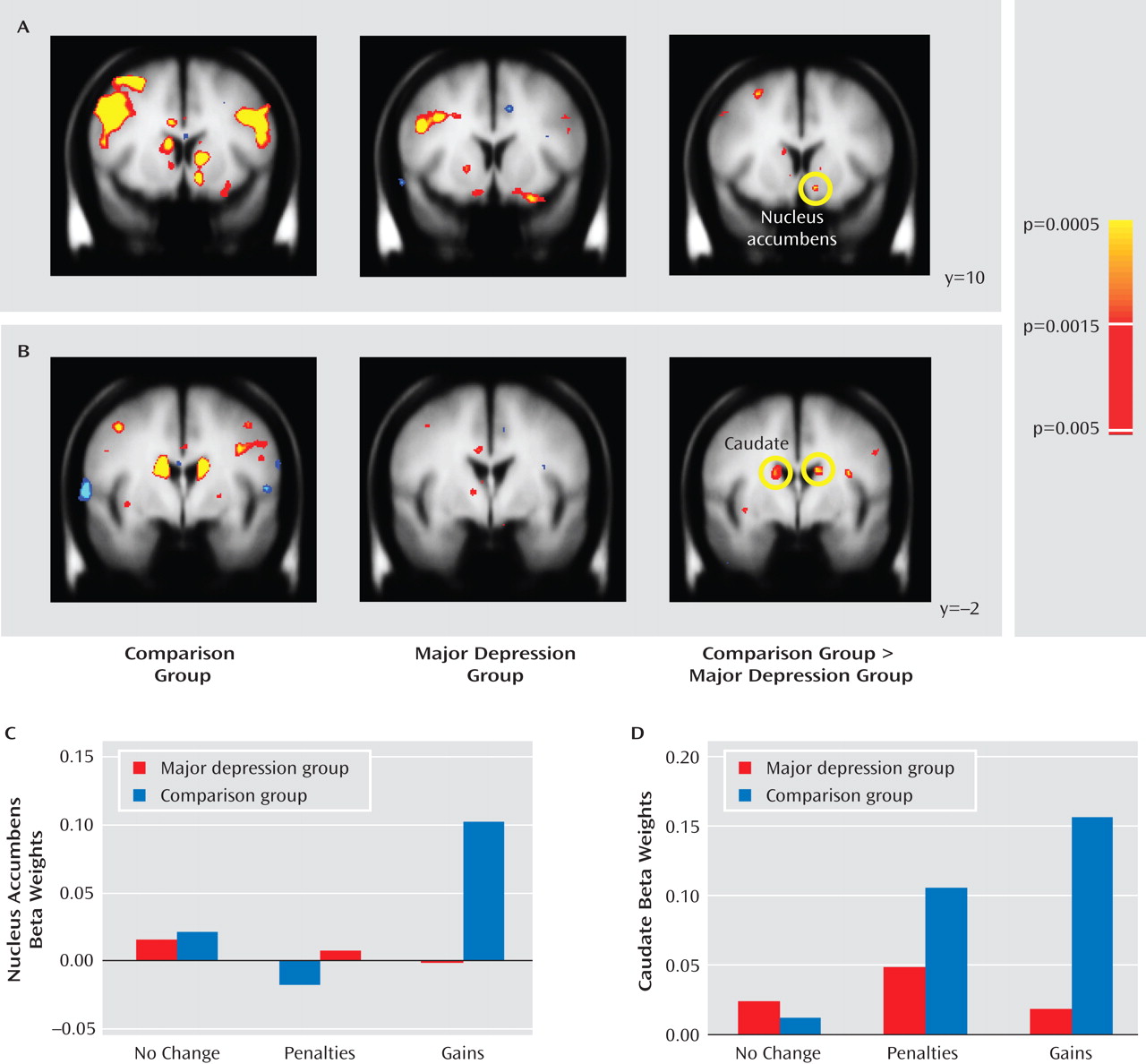
Relative to comparison subjects, the major depression group showed significantly weaker responses to gain versus no-change feedback in the left nucleus accumbens and the dorsal caudate bilaterally, including two subregions in the right caudate and two in the left caudate (
Figure 3 A and B). Both clusters in the right caudate and one in the left caudate remained significant after correction for multiple comparisons (see Table S4 in the online data supplement); accordingly, differences in the nucleus accumbens should be considered preliminary. To test whether group differences were specific to reward outcomes, mean beta weights were extracted from each cluster and entered into group-by-condition (gains, penalties, and no-change feedback) ANOVAs; for the caudate regions of interest, the factor subregion was added. For brevity, only effects involving group are reported.
In the accumbens (
Figure 3 C), a main effect of condition (F=3.46, df=2, 110, p<0.040) was qualified by the group-by-condition interaction (F=2.94, df=2, 110, p=0.063); the main effect of group was not significant. Because of a priori hypotheses regarding the accumbens, and given the significant group-by-condition interaction in the whole-brain analysis, follow-up tests were performed to clarify the source of the interaction. Relative to the comparison group, the major depression group showed significantly weaker responses to gains (p<0.005) but not to penalties or no-change feedback. Furthermore, within-group tests showed that while comparison subjects responded more strongly to gains compared with both penalties (p<0.004) and no-change (p<0.001) feedback, in participants with major depression left accumbens activation was not modulated by condition.
In the caudate (
Figure 3 D), the ANOVA revealed significant main effects of subregion, condition, and group (p values <0.013), a significant condition-by-subregion interaction and, most important, a significant group-by-condition interaction (F=7.89, df=2, 110, p<0.002). This interaction was due to significantly greater activation in the comparison versus the major depression group in response to gains (p<0.0002) but not to penalties or no-incentive feedback. Moreover, whereas comparison subjects showed increased caudate activation bilaterally in response to both gains and losses (p values <0.0002) relative to no-change feedback, participants with major depression failed to show any feedback-dependent caudate modulation. No correlations emerged between activation in the left putamen, left accumbens, or caudate and anhedonic symptoms in either group.
Morphometric Data
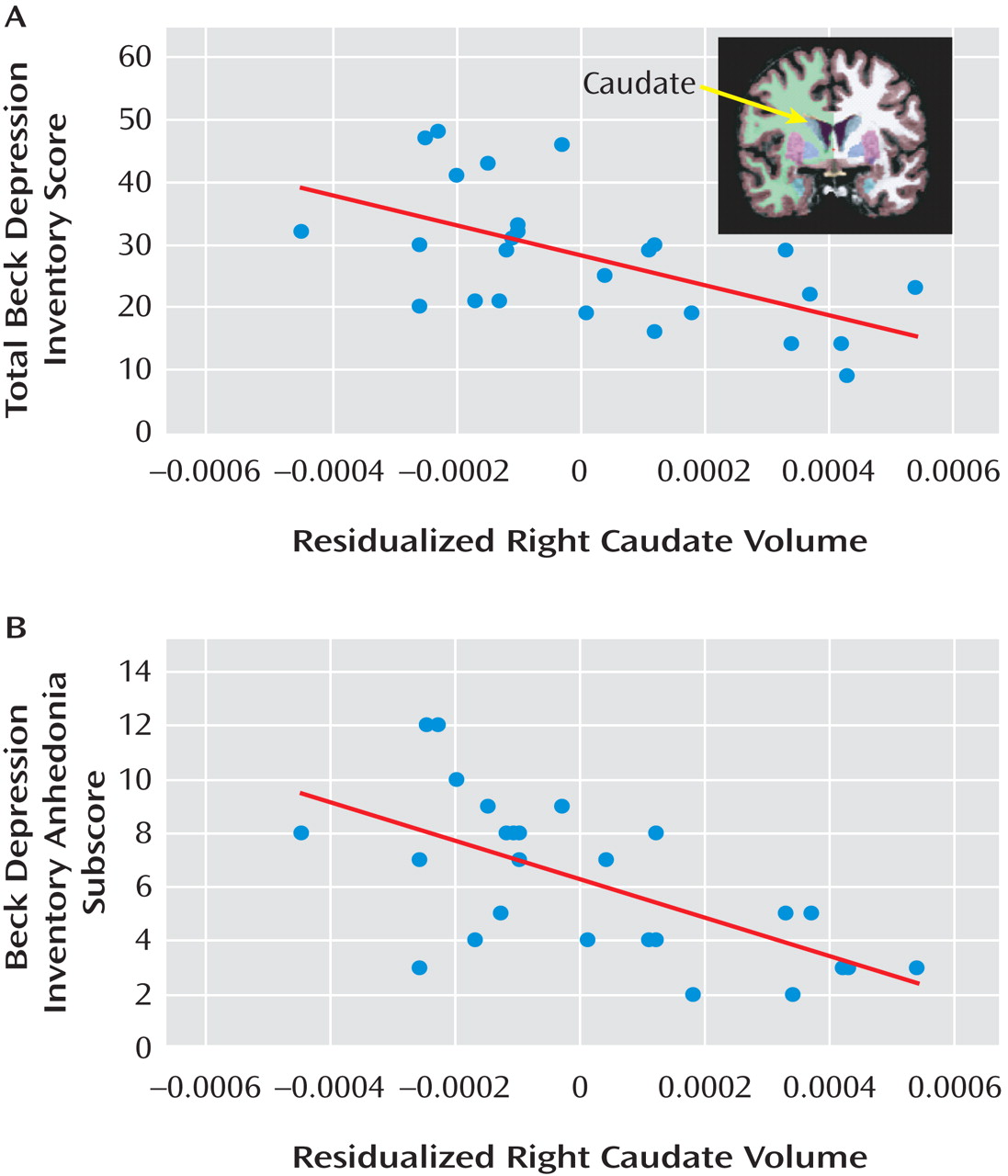
The group-by-hemisphere-by-region ANOVA revealed no group differences (see Table S5 in the online data supplement). In the major depression group, correlations were run between 1) proportional left accumbens and bilateral caudate volumes and 2) anhedonic symptoms and depression severity. For the left accumbens, no significant effects emerged. For the left and right caudate, volume was inversely related to total BDI score (left: r=–0.489, p<0.015; right: r=–0.579, p<0.002) and BDI anhedonia subscore (left: r=–0.553, p<0.004; right: r=–0.635, p<0.0001) (
Figure 4 ). Critically, both left and right caudate volumes predicted total BDI score and BDI anhedonia subscore after adjusting for age and gender (total BDI score: left caudate, ΔR
2 =0.203; right caudate, ΔR
2 =0.309; BDI anhedonia subscore: left caudate, ΔR
2 =0.281; right caudate, ΔR
2 =0.387; all ΔF >6.09, p values <0.025).
Control Analyses
In light of group differences in valence ratings for reward cues and valence and arousal ratings for gains, control analyses evaluated whether group differences in left putamen reward cue responses and left accumbens and bilateral caudate gain responses remained after controlling for affective ratings (see the online data supplement). Regression analyses confirmed that this was the case. Moreover, group differences in accumbens and caudate gain responses remained after controlling for the volumes of these structures and group differences in reward-related reaction time modulation. In addition, no significant correlations between reward-related accumbens and caudate activation and the volume of these regions emerged. Finally, there were no differences in basal ganglia activation for participants with major depression with comorbid anxiety (N=14) compared to those without (N=16).
Discussion
This study investigated anticipatory and consummatory phases of reward processing in depression. Behaviorally, the major depression group showed evidence of anhedonia, reporting generally reduced positive affect to reward stimuli and less arousal following gains. These findings were mirrored by group differences in basal ganglia responses to rewarding outcomes, as participants with major depression showed weaker responses to gains in the caudate bilaterally and in the left nucleus accumbens. By contrast, there was less evidence of differences during reward anticipation. Both groups showed robust basal ganglia responses to reward cues, and although comparison subjects activated the left posterior putamen more strongly than did participants with major depression, the size of the cluster was relatively small. Also, groups did not differ in reaction time as a function of cue, although relatively weaker modulation by reward was seen in participants with major depression (see difference scores). Finally, negative correlations between anhedonic symptoms (and depression severity) and caudate volume emerged in participants with major depression. These findings, which extend previous reports of basal ganglia dysfunction in major depression
(6 –
11,
30), suggest that this dysfunction is more closely associated with consummatory than anticipatory deficits and emphasize a role for reduced caudate volume in anhedonia.
Reduced Basal Ganglia Response to Rewarding Outcomes in Major Depression
The strong caudate response to gains in comparison subjects fits human
(18,
20,
31) and animal
(32) studies demonstrating this structure’s sensitivity to reward-related information. The caudate responds maximally when rewards are unpredictable (e.g., when delivered on 50% of reward trials, as was done here) and subjects believe that outcomes are contingent on their actions
(31) . Accordingly, the between-group caudate difference suggests a weaker perceived action-outcome relationship and/or weaker responses to unpredictable rewards in depression.
Evidence for the first interpretation is mixed. Although groups differed in reward-related reaction time modulation (reaction time difference scores), there was no group difference in reactions on reward trials, and both groups responded faster on reward trials than on loss or no-incentive trials. Thus, both groups behaved as though their responses influenced the chances of receiving gains. Alternatively, the impact of the gains may have been weaker in participants with major depression. This is consistent with the fact that participants with major depression reported overall blunted affective responses and decreased arousal to gains. In addition, group differences were also observed in the left nucleus accumbens, a region that responds strongly to rewarding stimuli
(33) . Activity in the accumbens appears to track the hedonic value of outcomes
(31,
34) . Thus, while the group difference in caudate responses suggests a depression-related deficit in expressing goal-directed behaviors, the finding in the accumbens indicates a more primary deficit in hedonic coding. These results are consistent with evidence indicating that deep brain stimulation to the accumbens
(35) and ventral capsule/ventral striatum
(36) significantly reduced symptom severity and anhedonia in treatment-resistant patients with major depression. Collectively, these findings indicate that dysfunction in regions mediating hedonic impact (accumbens) and reinforcement of actions (caudate) plays an important role in the pathophysiology of major depression.
The group differences in gain responses are intriguing in light of reports of reduced ability to modulate behavior as a function of intermittent rewards in major depression
(5) . Using a probabilistic reward task, we found that depressed subjects, particularly those reporting anhedonic symptoms, showed a reduced response bias toward a more frequently rewarded stimulus relative to comparison subjects. Furthermore, healthy comparison subjects with blunted response bias in the probabilistic task also generated weak basal ganglia responses to gains in the fMRI task used here
(37) . These considerations suggest that weak basal ganglia responses to unpredictable rewards may contribute to poor learning of action-reward contingencies in major depression.
Intact Basal Ganglia Responses to Reward Cues in Major Depression
Surprisingly, both groups showed robust basal ganglia responses to reward cues. However, in contrast to results in a prior study
(17), the major depression group in the present study showed weaker reward-related reaction time modulation and affective responses to reward-related stimuli relative to comparison subjects. Thus, behavioral evidence of reward processing deficits can coexist with significant basal ganglia responses to reward-predicting cues.
The nature of the intact basal ganglia response to reward cues in individuals with major depression is unclear. In incentive delay tasks, anticipatory ventral striatal activity is typically regarded as related to the dopamine signal seen in response to reward cues in electrophysiological studies
(38) . In nonhuman primates, this signal is first elicited by unpredicted rewards and travels back to cues only when a cue-outcome contingency is learned
(14) . In our study, the comparison group showed a significantly stronger basal ganglia response to gains than the major depression group, yet the two groups showed few differences in response to reward cues. This suggests two possibilities: 1) the unlikely possibility that the dopamine signal traveled from the gains (consummatory phase) to the cues (anticipatory phase) more rapidly in individuals with major depression or 2) the more likely possibility that the reward cues elicited a ventral striatal response on their own that was similar across groups and possibly independent of transmission of the dopamine signal elicited by gains. This possibility is rarely considered in studies using incentive delay tasks, but because participants know that reward cues can lead to gains, it is possible that the cues elicit ventral striatal activation from the outset. However, even if this is the case, a group difference in ventral striatal response to reward cues might still be expected
(8) . Studies in which participants learn cue-reward associations over time are needed to investigate this issue.
Reduced Caudate Volume and Anhedonia
Replicating findings with nonclinical subjects
(23), participants with major depression who had elevated anhedonic symptoms showed reduced caudate volumes bilaterally. This relationship provides impetus for continued investigation of depressive endophenotypes
(1,
2), because it is unclear whether reduced caudate volume predisposes individuals to anhedonic or more severe depression or instead represents a state-related correlate of these symptoms.
Limitations
Several limitations of this study should be emphasized. First, although the a priori hypothesis about the nucleus accumbens
(8,
10,
11) was confirmed by a group-by-condition interaction at p<0.005, this difference was not significant after correction for multiple comparisons because of the small cluster size (see the online data supplement). Moreover, no correlations between striatal activation and anhedonic symptoms emerged. Consequently, additional studies are needed to confirm the role of the nucleus accumbens in reward dysfunction in major depression. Given mounting interest in the role of the accumbens in the pathophysiology of major depression, as exemplified by recent deep brain stimulation studies targeting this region
(35,
36), our finding of reduced reward-related accumbal responses is nevertheless intriguing. Second, correlations between caudate volume and depression severity emerged for BDI score but not HAM-D score. Although the reason for this discrepancy is unclear, it is possible that several BDI items tapping anhedonia contributed to this finding. In spite of these limitations, the current findings indicate that anhedonia, a core component of major depression, may reflect weak reward consummatory responses in the basal ganglia, particularly the nucleus accumbens and the caudate, and is related to reduced caudate size.