Genetic variants that contribute to the risk of psychiatric disorders may also alter neurobehavioral phenotypes in healthy individuals
(1) . The catechol-
O -methyltransferase (COMT) gene on the chromosome region 22q11 is an a priori candidate for variation in both normal and abnormal psychological function because it is instrumental in the synaptic catabolism of dopamine and other catecholamine neurotransmitters
(2) .
Associations have been reported between COMT valine-to-methionine (Val
158 Met) and a diverse range of psychiatric and psychological phenotypes. Although the Val
158 allele and high expression haplotypes may confer risk of schizophrenia
(2,
3), association studies of this disease have produced many conflicting results, and overall the contribution to disease risk is small at best
(4) . Similarly, the COMT Met
158 allele is more strongly predictive of enhanced brain metabolic responses to emotion and pain
(5) than it is to trait anxiety
(6) or anxiety diagnoses, where effects are modest
(7,
8) . Indeed, the low and high activity alleles of the common COMT Val
158 Met polymorphism appear to have counterbalancing effects in the normal behavioral domains of cognition and emotional response, explaining the very high frequency (0.3–0.7) of both alleles in populations worldwide, as summarized by the “warrior/worrier” explanatory model
(9) .
The diverse effects of COMT on behavior might be expected because of the widespread expression of this enzyme in the brain and its role in the metabolism of catecholamine neurotransmitters. However, genetic variation in this enzyme is likely to be particularly important for the diverse disorders and behaviors associated with function of the prefrontal cortex. Recent in vivo studies in COMT knockout mice suggest that COMT-mediated enzymatic degradation is responsible for one-half of dopamine decline in the prefrontal cortex
(10) .
The COMT protein exists in the following two forms arising from distinct start sites: 1) the soluble form (S-COMT), which predominates in the peripheral nervous system, and 2) the membrane-bound form (MB-COMT), which is more abundant in the brain
(11) . An extensively studied single nucleotide polymorphism (SNP) in exon 4 of MB-COMT results in a Val
158 Met substitution (rs4680), which alters the enzyme’s thermostability
(12) . The Met
158 substitution leads to approximately 40% lower COMT activity at body temperature
(13) .
Neuroimaging studies confirm that COMT genotype affects human prefrontal cortical function. On a range of prefrontal cognitive tasks, Val/Val individuals show consistently greater activation in the dorsolateral prefrontal cortex than Met carriers at equivalent levels of performance
(2,
14), presumably reflecting increased cortical efficiency in Met carriers
(2) . The Met advantage can be disrupted by administration of a single dose of amphetamine
(15), which improves performance in Val/Val individuals but appears to impair performance in Met/Met individuals. This presumably results from moving Met/Met carriers over the apex of the “inverted-U” shape function of optimal dopaminergic signaling
(16) .
The increased cortical efficiency associated with Met carriers in neuroimaging experiments only indirectly and less distinctly translates to improved cognitive performance. For example, a recent meta-analysis
(17) observed no significant effect of the Val
158 Met SNP on frontal cognitive tasks. Possible reasons for this include heterogeneities in task and sample across studies. An alternative explanation is that the cognitive action of Val
158 Met is confounded by effects of other polymorphisms within COMT, which affect the level of gene expression and are in partial, but not complete, linkage disequilibrium with Val
158 Met. The importance of several such noncoding SNPs has been convincingly demonstrated by functional studies. In the most comprehensive account of COMT function, Nackley et al.
(18) described a haplotype block defined by Val
158 Met, SNP rs6269 in the P1 promoter, and two synonymous SNPs, rs4633 in exon 3 and rs4818 in exon 4. The greatest differences in COMT expression were reported between haplotypes divergent in the two synonymous SNPs, which together accounted for 9% of variance in a pain sensitivity phenotype. COMT enzymatic activity in transfected rat adrenal cells showed 18- to 25-fold differences across haplotypes, which were ascribed to differences in the stability of mRNA secondary structure
(18) .
Despite solid evidence for the biological importance of these SNPs, little is known about the cognitive effects of any loci other than Val
158 Met. Previous studies have reported associations between an SNP in the 3′ untranslated region (rs165599), previously associated with schizophrenia
(19), and cognitive dysfunction in schizophrenia
(20) and bipolar disorder
(21) . However, a large study of patients with schizophrenia and related and unrelated comparison subjects
(22) found no association between rs165599 and multiple measures of working memory or executive function. To the best of our knowledge, there is no specific report of the effects of the Nackley et al. haplotype on cognitive function, although one recent study
(23) reported better planning ability and worse performance on the Iowa Gambling Task among healthy men with the CC genotype at rs4818, a SNP which tagged the functional haplotype in the Nackley et al. study.
We previously investigated sex-specific cognitive effects of Val
158 Met in a large population-based sample of U.K. children
(24) . Boys but not girls with the Met allele performed better on measures of verbal IQ and working memory. In the present study, we extended this research in the same sample to investigate the cognitive effects of four other COMT SNPs implicated in psychological functioning, including SNPs defining the Nackley et al. haplotype
(18) . We also investigated two secondary hypotheses. Based on the COMT literature, we investigated 1) whether the genetic effects were sexually dimorphic
(25) and 2) whether they were linear or followed an inverted-U shape
(15) .
Method
Sample
The Avon Longitudinal Study of Parents and Children
(26) is a general population cohort based in South West England. The cohort consisted of 14,062 live births from 14,541 enrolled pregnant women who were expected to give birth between April 1, 1991, and December 31, 1992. From age 7, all children were invited for an annual assessment of physical and psychological development, and up to 8,000 children continue to attend each year. The present study concerns cognitive assessments undertaken at the age 8 and 10 years assessments (see reference
25 ). To increase sample homogeneity, only children of Caucasian ethnicity (95% of the cohort) were included in the study. Parents gave informed consent at enrollment, and ethical approval was obtained from the Avon Longitudinal Study of Parents and Children and local research ethics committees.
Cognitive Assessment
IQ was assessed at age 8 (mean=8 years, 8 months [SD=3.1 months]) using the WISC-III (U.K. edition)
(27) . Verbal and performance IQ scores were calculated in accordance with standard procedures whenever four subscale scores were available. At the same time, verbal inhibition was assessed by the time taken to perform the Opposite Worlds task
(28) . This task requires the child to read aloud a string of the digits 1 and 2 and respond in the “opposite” manner (i.e., saying “one” for the digit 2). At age 10 (mean=10 years, 8 months [SD=3.0 months]), visual working memory was assessed using the Count Span task
(29) . In this task, the child must count out loud the number of red dots presented on a screen. After viewing multiple screens, the child is asked how many dots were on each screen within that set and receives a span score based on the number of correctly recalled sets, with a maximum score of 5 in increments of 0.5.
Genotyping
Genotyping was performed as previously described
(6) . DNA was extracted from blood using standard methods, and 5′ nuclease assays were used to genotype the following five SNPs: rs2075507 (previously rs2097603), rs6269, rs4818, rs4680, and rs165599. Primer/probe combinations (see the data supplement accompanying the online version of this article) were designed using Primer Express software 2.0 (ABI, Foster City, Calif.). DNA sequences were obtained from GenBank and the Celera Discovery System. Allele discrimination was performed using a Taqman 7900 machine. Two standards for each genotype were confirmed by sequencing six genomic DNA samples using a 377 sequencer (ABI, Foster City, Calif.).
Analysis
Of the five genotyped SNPs, the central three (rs6269, rs4680, and rs4818), which define the Nackley et al. haplotype
(18), were in strong linkage disequilibrium (all d′ >0.85). Linkage disequilibrium patterns from additional markers in the region (data from HapMap) were used to determine that there are three major cladistically related haplotype groups, each containing one or more common haplotype (see the data supplement accompanying the online version of this article). The two haplotypes containing Val
158 correspond to higher and lower expressing COMT genetic backgrounds, with ValA having both the highest COMT enzyme activity and the highest mRNA expressing haplotype
(18) . In contrast, linkage disequilibrium between the three SNPs in the haplotype and the other two SNPs was low (all d′ <0.5 for rs2075507 and <0.3 for rs165599). We therefore chose to separately test associations between cognitive scores and rs2075507 and rs165599 and with the three-SNP haplotype.
In the main analyses, the SNPs rs165599 and rs2075507 were independently tested for association with cognitive phenotypes using one-way analysis of variance (ANOVA). The three-SNP Nackley et al. haplotypes were derived using PHASE
(30), and subjects were assigned to one of six possible diplotypes. These were ranked from highest to lowest COMT activity according to known functionality as follows: ValA/ValA, ValA/Met, ValA/ValB or Met/Met, ValB/Met, and ValB/ValB. Cognitive scores were compared between these five categories by one-way ANOVA.
To test whether the genetic effects were best characterized as linear or curvilinear (inverted-U), linear and quadratic regressions were fitted for each analysis and the variance explained by each was compared. To assess potential sex-by-genotype interactions, we analyzed each cognitive outcome using general linear models with main effects of sex and genotype (linear and quadratic components) and a sex-by-genotype interaction term.
Power calculations indicated that we had 90% power to detect a genetic variant accounting for just 0.2% of variance in the cognitive score in the overall sample at α=0.05 under an additive model. All cognitive variables were normally distributed according to one-sample Kolmogorov-Smirnov test except verbal inhibition trial time, which was transformed (1/√ msec) to allow parametric analysis. Sample size varied between analyses according to the availability of cognitive data. Minimum sample size for each analysis is shown in
Table 1 . Deviation from Hardy-Weinberg equilibrium and associations between sex and genotype were assessed using chi square tests.
Results
Genotyping was completed in 8,173 children (4,211 boys; 3,962 girls), and cognitive scores were available for two-thirds of these. Allele and haplotype frequencies for each SNP were as previously reported (see reference
6 and the data supplement accompanying the online version of this article). All frequencies were consistent with Hardy-Weinberg equilibrium. Chi square tests showed no association between any SNP and sex (all p values >0.1). This test was performed because we evaluated gene-by-sex interaction.
Genetic Effects on Cognition
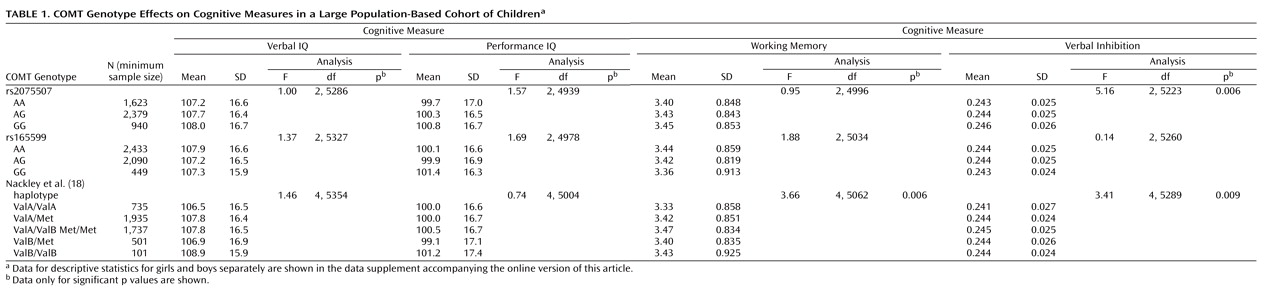
Table 1 shows descriptive statistics and ANOVA results for each genetic analysis. One-way ANOVA showed no effects of rs165599 on any cognitive measure. For rs2075507, there was a significant effect only on verbal inhibition (also see the data supplement accompanying the online version of this article), such that better verbal inhibition was associated with higher dopamine availability
(13) . For the five-group diplotype, there were significant effects on verbal inhibition and on working memory but not on verbal or performance IQ. Separate scores for boys and girls are shown in the data supplement accompanying the online version of this article.
Linear and Nonlinear Genetic Effects of COMT Diplotype
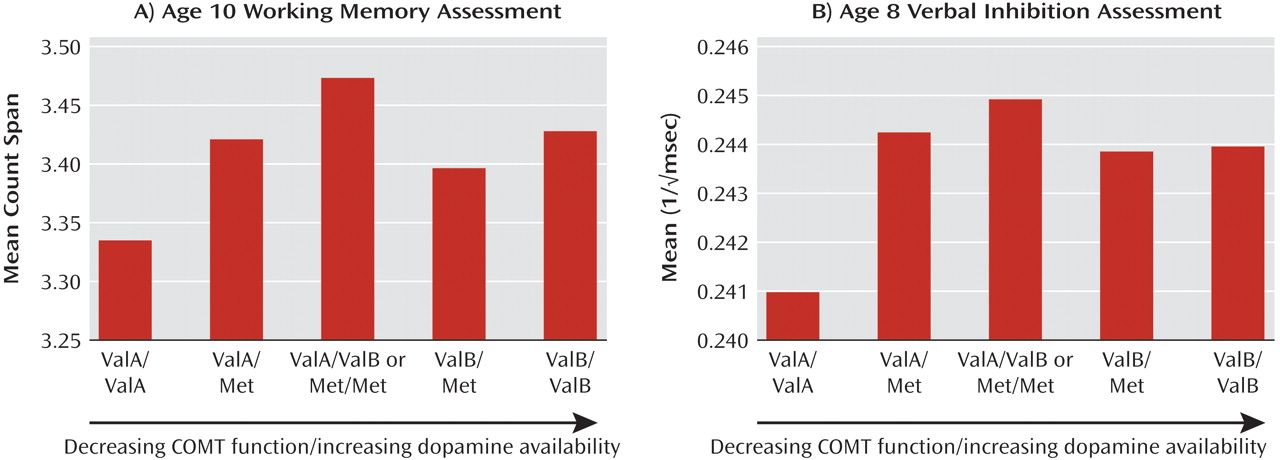
For the genetic effects described for the Nackley et al. diplotype, adding a quadratic term contributed significantly to the fit of the regression model and explained a greater proportion of variance in the cognitive score than did a linear model. For the working memory model, fit was greater in the quadratic model (F=6.13, df=2, 5064, p=0.002) than in the linear model (F=4.89, df=1, 5065, p=0.03), and both linear (β=0.19, t=3.15, p=0.002) and quadratic (β=–0.17, t=–2.72, p=0.007) terms were significant in the quadratic model. For verbal inhibition, model fit was also greater in the quadratic model (F=6.22, df=2, 5291, p=0.002) than in the linear model (F=5.29, df=1, 5292, p=0.02), and again, both the linear (β=0.19, t=3.13, p=0.002) and quadratic (β=–0.16, t=–2.67, p=0.008) terms in the quadratic model were significant (
Figure 1 ). In both cases, r
2 increased from 0.001 to 0.002 between the linear and quadratic models.
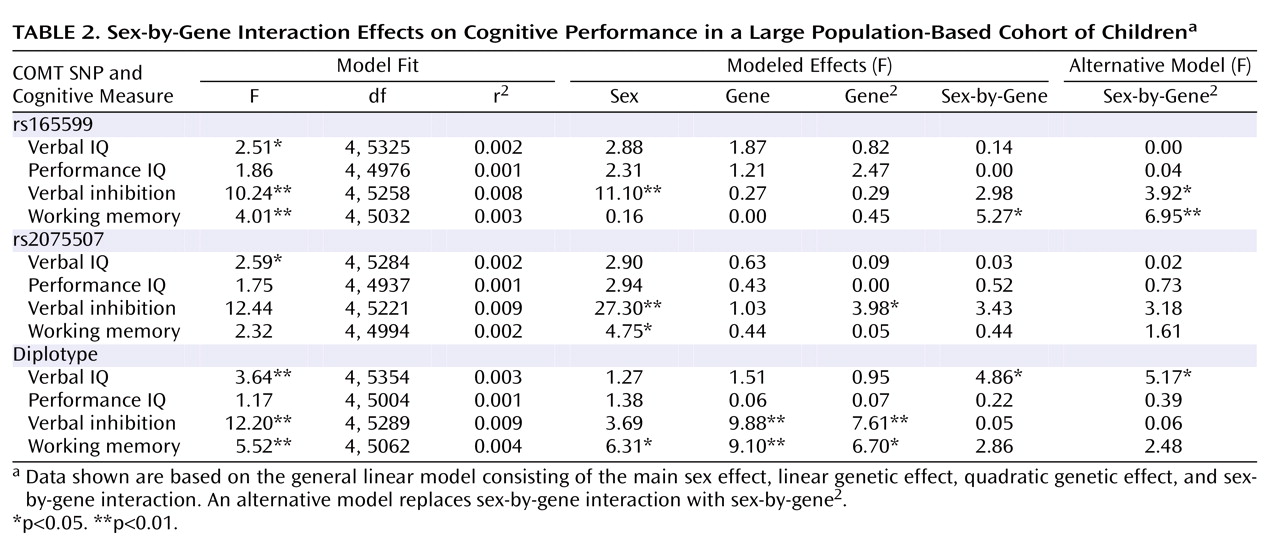
Sex-By-Gene Interactions
To test sex-by-genotype interactions, we applied general linear models containing main effects of sex, genotype (linear and quadratic components), and a sex-by-genotype interaction term. There was little difference in model fit or effects whether the sex-gene interaction was modeled as a linear or quadratic term (
Table 2 ), but for ease of interpretation we have provided the linear interaction as our primary model. Main effects of genotype and sex were consistent with the effects reported in the present article as well as previously
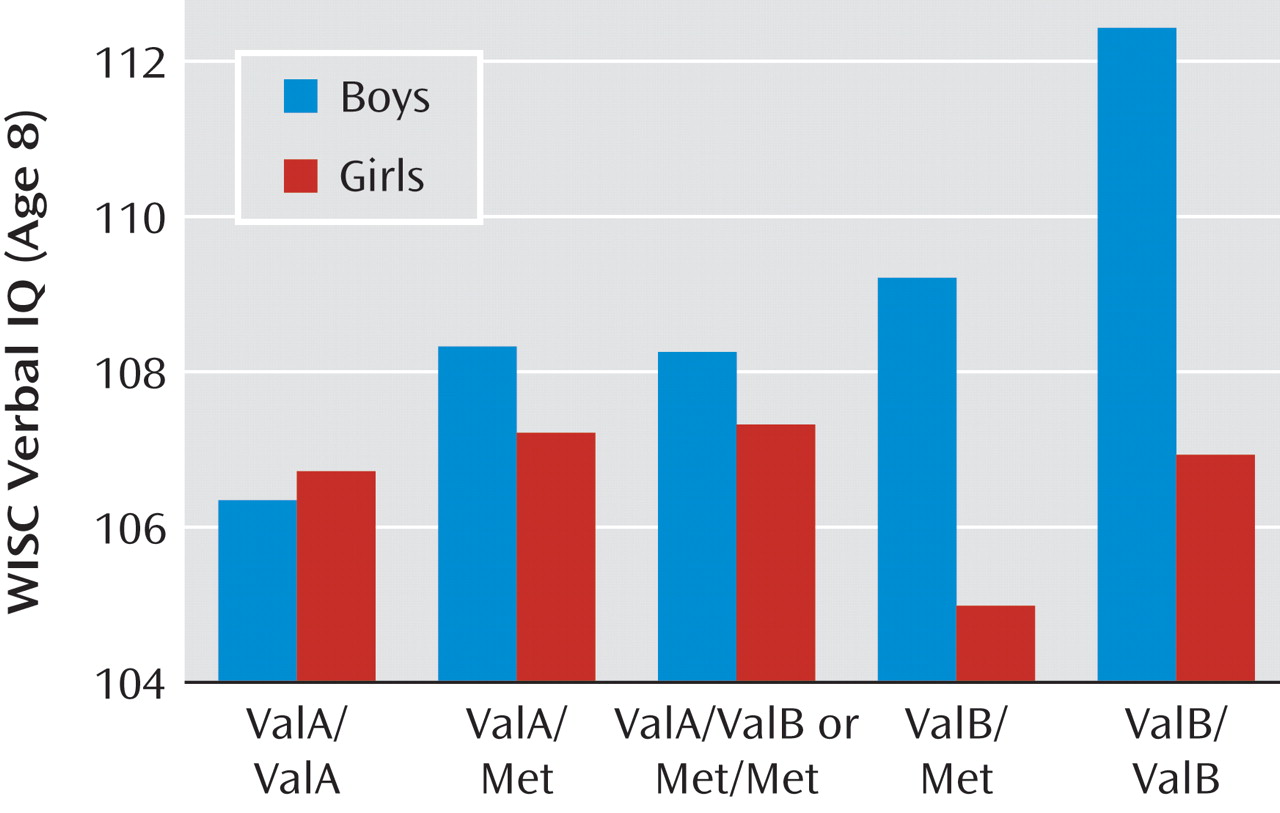
(25) . There were significant sex-by-genotype interaction effects for verbal IQ with the Nackley et al. diplotype (F=4.86, df=1, 5354, p=0.03 [
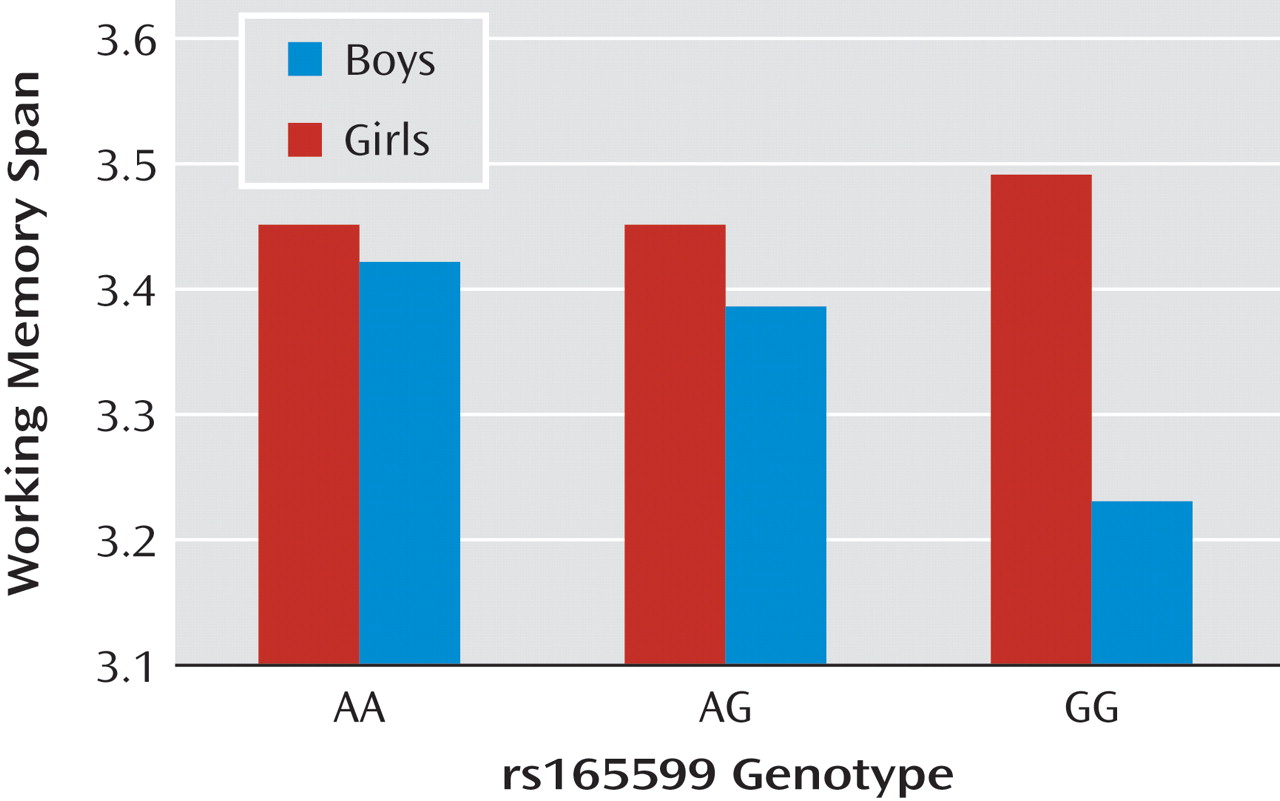
Figure 2 ]) and for rs165599 and working memory span (F=5.27, df=1, 5302, p=0.02 [
Figure 3 ]), and there were marginal effects for both rs165599 (F=2.98, df=1, 5258, p=0.08) and rs2075507 (F=3.43, df=1, 5221, p=0.06) and verbal inhibition.
Discussion
Inconsistent replication has been the pattern for the many psychological and psychiatric associations reported for COMT. It has recently been demonstrated that loci other than Val
158 Met are crucial in understanding the genetic regulation of COMT
(18) . To the best of our knowledge, the present study represents the largest and most complete survey of the cognitive effects of COMT, suggesting that although Val
158 Met does influence normal variation in cognitive function, other SNPs that affect the level of COMT expression play important additional roles.
We found evidence for association between rs2075507 in the P2 promoter and verbal inhibition and no main cognitive effects for rs165599 in the 3′ untranslated region except a sex-by-gene interaction with working memory. Despite relatively little prior evidence regarding cognition, the known functional effects of these SNPs support these results. Both rs2075507 and rs165599 have previously been reported to interact with Val
158 Met in predicting prefrontal cortical response during a working memory task, appearing to modulate Val
158 Met-related brain activity in a nonlinear fashion
(31) . Moreover, both SNPs have also been implicated in risk haplotypes for psychiatric disorders, particularly schizophrenia
(3,
19,
32) . Although this is the first study to assess the cognitive effects of rs2075507, there are three prior reports on the cognitive effects of rs165599. Two of these studies
(20,
21) found a cognitive decrement associated with the G allele of rs165599, which appeared to be found in boys in our study (
Figure 3 ). One larger study
(22) found no overall effect, consistent with the lack of main effect in our sample, and there have been no prior reports with the opposite direction of effect. Taken together, these reports suggest that potential sex-specific cognitive effects of rs165599 warrant further investigation.
Verbal IQ showed no main genetic effect except a significant sex-by-haplotype interaction, such that there appeared to be a linear relationship between haplotype and verbal IQ in boys and no association in girls (
Figure 2 ). In contrast, performance IQ was not predicted by the Nackley et al. haplotype (nor by any other COMT SNP). In our prior investigation in this sample
(25), the largest effects of Val
158 Met on cognition were found for verbal IQ. It is interesting that the other SNPs investigated in the present sample showed effects on working memory and verbal inhibition but not on IQ measures and the mean difference in verbal IQ between highest and lowest activity diplotypes in boys was approximately six points (
Figure 2 ), which was double that found when the same sample was classified by Val
158 Met alone
(24) . Importantly, the group with the highest mean verbal IQ was the small proportion of boys characterized by the ValB/ValB diplotype (lowest enzymatic activity). When classified by Val
158 Met alone, these boys would be denoted Val/Val (the highest activity group). This important discrepancy, plus the possibility of sex interactions, may help explain inconsistencies in previous reports of associations between cognition and the Val
158 Met SNP.
We found evidence for statistically significant interactions between genotype and sex in two analyses and marginal evidence in two further analyses. Given that the four measures of cognition are to some extent correlated (r=0.3–0.5), strict control for multiple comparisons (for example, using Bonferroni corrections) would result in an overly conservative criterion. Had we applied this correction, it is interesting to note that all main genetic effects would have remained significant (i.e., all p values <0.01), but the sex-by-gene interaction terms would have been nonsignificant at this level. As such, the sex-by-gene effects should perhaps be considered more tentative. Nonetheless, they are supported by the emerging body of evidence that COMT’s effects are sexually dimorphic
(25) . COMT activity in human postmortem prefrontal cortex tissue is approximately 17% higher in men than women
(13), and frontal cortical dopamine levels are affected in male but not female COMT knockout mice
(33) . The presumed explanation for this sexual dimorphism is the bidirectional relationships between COMT and estrogen-related compounds. Estrogens mediate COMT expression
(34), while COMT metabolizes catechol estrogens, a process which is probably regulated by the Val
158 Met SNP
(35) .
Both working memory and verbal inhibition were associated with the Nackley et al. haplotype in a nonlinear manner consistent with an inverted-U model of dopamine function. Studies in humans and other species
(36,
37) have demonstrated that there is an inverted-U shape relationship between prefrontal dopaminergic function and working memory performance, such that both hypo- and hyperdopaminergic function are detrimental to working memory. Previous studies of the COMT Val/Met polymorphism have suggested that the high prefrontal dopamine availability associated with the Met can be detrimental to cognitive function under circumstances including acute amphetamine administration
(15) and perhaps also within the range of normal brain function
(38) . Our data support the view that this inverted-U function is reflected in normal variation in cognitive performance when multiple COMT SNPs, which together influence both COMT activity and COMT expression, are taken into account.
The effects reported in the present study are small, explaining between 0.2% and 0.4% of variance in cognitive function. This magnitude of effect size is plausible given both our best estimates of COMT’s effects from meta-analysis of the current literature
(17) and the likely magnitude of individual genetic effects resulting from genomewide association studies of psychological traits (e.g.,
39 ). The lack of statistical power may explain why we detected genetic effects on some cognitive measures but not others. It is possible that individual SNPs have even smaller effects than we were able to detect (i.e., <0.2% trait variance). More likely is that the relatively simple cognitive measures used in the present study may be uneven assays of COMT’s cognitive effects, which may depend not only on the relative importance of dopaminergic and other monoamine substrates of the task but also on a complex relationship between cortical and subcortical dopaminergic signaling
(40) .
Although relatively well studied, COMT is not a simple gene, and intense scrutiny by the field of psychiatric genetics has merely increased our understanding of its complexity. Recent studies have shown that COMT gene products include multiple mRNA variants expressed at different levels throughout the brain
(41), throughout development
(42), and with tissue-specific monoallelic expression
(43) . The nonlinear and sex-specific effects suggested by the present study—while complicated—may yet be only a step toward describing the true complexity of effects that characterizes this gene.