Autism is defined by the presence of markedly abnormal development in social interaction, communication, and repertoire of activity and interests (DSM-IV). The dynamic neurological and social deficits observed in children with autism contribute to varying levels of impairment. Although advancements in autism research have improved our understanding of the disorder, many questions regarding the pathophysiology of autism remain
(1) .
Two decades of magnetic resonance imaging (MRI) studies have yielded somewhat consistent findings of specific brain regions that are altered in children with autism, including the frontal lobe
(2 –
4), amygdala
(1,
5 –
11), cerebellar vermis
(12 –
21), and corpus callosum
(14,
22 –
27) .
Studies indicate that young children with autism initially exhibit brain enlargement, followed by a decrease in overall brain volume over time. Brain enlargement is most pronounced in the frontal lobe
(28), possibly as a result of the late development of this region
(29) . However, the specific neuroanatomic subregions that contribute to growth dysregulation of the frontal lobe have not been firmly established. Enlargement has been observed in dorsolateral and medial frontal regions
(2) but not in the orbitofrontal cortex
(3) of children with autism. It has been suggested that possible alterations in neuronal development of the frontal cortex in autism ultimately may lead to abnormal local and long-distance neural connectivity
(29) . Supporting this hypothesis, in part, are diffusion tensor and anatomic imaging studies of the corpus callosum, in which reductions in fractional anisotrophy and/or volumes have been observed in both anterior and posterior aspects of this structure in individuals with autism
(23 –
26,
30,
31) .
Less consistent are MRI studies of the cerebellar vermis in autism, some of which have observed vermal hypoplasia
(12), particularly in lobules VI and VII, and others have observed vermal hyperplasia
(13) . Variability in findings are most likely attributable to the heterogeneity of the disorder
(13) .
Similarly, studies of alterations of the amygdala in autism have been inconsistent. Results vary between increased
(7,
9,
32), decreased
(5,
6), and same-sized amygdala volumes
(33) . One possible explanation for these seemingly contradictory findings is the variability in age and degree of severity across autistic subject populations. Further, a severity-by-age interaction has been proposed, suggesting that younger less affected autistic subjects would have normal to enlarged amygdala volumes, while older more affected individuals would have smaller amygdala volumes
(10) .
Several lines of research suggest that genetic, epigenetic, and environmental interactions structure the brain
(34) and therefore contribute to autistic deficits as well as accompanying neuroanatomic anomalies. Studying monozygotic twins with autism who may be discordant for the disorder can elucidate the respective influences of genetic and environmental factors on susceptibility to autism. In a previous study of neuroanatomic variation in a cohort of monozygotic twin pairs discordant for the narrow phenotype for autism
(4), we used a semiautomated method to measure CSF and gray and white tissue compartments of lobar regions. We found that relative to typically developing comparison subjects, both children with autism and their co-twins exhibited decreased volumes in frontal, temporal, and occipital white matter. In order to extend the findings of our previous study, which did not focus on sublobar regions, we conducted manual volumetric measurements of the total frontal and prefrontal cortices, corpus callosum, amygdala, hippocampus, and cerebellar vermis in this cohort. Based on previous research, we hypothesized that 1) volumes of the dorsolateral and dorsomedial cortical subregions and the amygdala would be enlarged; 2) areas/volumes of the corpus callosum and lobules VI and VII of the cerebellar vermis would be decreased in children with autism (although not necessarily their co-twins); and 3) the volumes of those regions that significantly differ between children with autism and comparison subjects would be associated with behavioral measures of severity of autism.
Previous neuroanatomic studies of typically developing monozygotic twins suggest a high degree of concordance in brain volumes. Giedd and colleagues
(35) performed multivariate analyses on neuroanatomic brain volumes of pediatric monozygotic and same-sex dizygotic twin pairs with typical development. They observed within-structure cross-twin correlations that were substantially higher for monozygotic twins relative to dizygotic twins. A pediatric imaging study of monozygotic and dizygotic twins conducted by Wallace and colleagues
(36) reported monozygotic correlations of gray and white matter volumes from different neuroanatomic regions of the brain that were nearly twice as large as dizygotic volumetric correlations of the same regions. In our previous study, we found that cerebral, but not cerebellar, gray and white matter volumes were highly correlated for both clinically concordant and discordant twin pairs. Considering this evidence, we would expect this same cohort to have highly concordant frontal lobe, corpus callosum, hippocampus, and amygdala volumes but not highly concordant cerebellar vermis volumes, suggesting a higher degree of genetic control over cerebral development relative to cerebellar development.
Method
Research Subjects
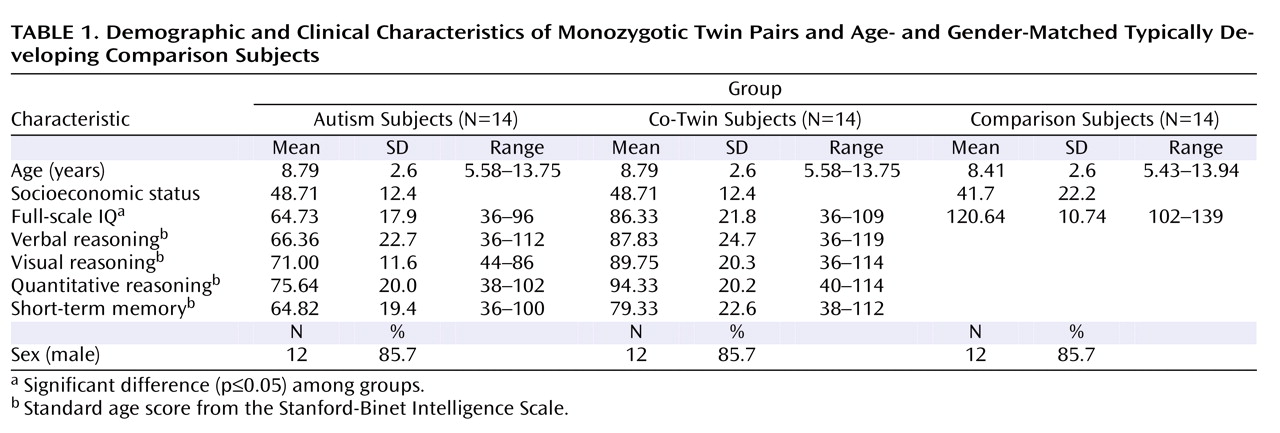
Participants were 42 children between the ages of 5 and 14 years old (14 pairs of monozygotic twins and 14 age- and gender-matched typically developing comparison subjects). The mean age of the twin pairs was 8.8 years (SD=2.6 years; range=5.6–13.8). The mean age of the comparison subjects was 8.4 years (SD=2.6 years; range=5.4–13.9). Twelve twin pairs and their comparison subjects were boys.
Families of children with autism were recruited through the Autism Society of America, the National Alliance for Autism Research, the Kennedy Krieger Center for Autism and Related Disorders, and clinical neurologists at the Kennedy Krieger Institute. Recruitment methods are described in detail elsewhere
(4) . In order to qualify for a diagnosis of autism, a child had to meet criteria for autism on the Autism Diagnostic Interview—Revised
(37) and score within one point of the criteria for autism on the Autism Diagnostic Observation Schedule—Generic
(38) . Each monozygotic twin pair in the study consisted of at least one child with a diagnosis of autism based on these criteria.
DNA fingerprinting probes were used to confirm zygosity in all twin pairs. Eight independent loci were tested for each twin pair. The DNA profiles for all twin pairs were statistically identical at every locus, indicating that the probability of monozygosity for each twin pair was approximately 99.99%.
Typically developing singleton comparison subjects were recruited from the office of a local pediatrician. Developmental diagnoses, which included language delay and psychiatric diagnoses (e.g., attention deficit hyperactivity disorder), were ruled out by parental report and the Child Behavior Checklist
(39) . None of the comparison subjects had known neurologic, developmental, learning, or psychiatric disorders.
MRI Pulse Sequence
MRI was performed on the same 1.5 Tesla GE Signa scanner (General Electric, Milwaukee). A three-dimensional high-resolution MRI scan was acquired for each subject using a T1-weighted spoiled gradient-echo sequence (time to repeat=35–45 msec, echo time=5–7 msec, number of excitations=1, flip angle=45°, matrix size=256×128, field of view=20–24 mm, yield=124 coronal slices, slice thickness=1.5 mm).
Measurement Protocols
Images were imported into the imaging software program BrainImage
(40) to remove nonbrain tissue and to measure the amygdala, hippocampus, and corpus callosum. Subsequently, images were moved to the software program Measure for measurement of the frontal lobe, subregions of the prefrontal cortex (dorsolateral, dorsomedial, orbitolateral, and orbitomedial), and the cerebellar vermis. Measures of all brain regions were based on previously published protocols
(41 –
45) . Raters were blind to diagnosis. Reliability coefficients were calculated for all regions of interest with the intraclass correlation coefficient, and raters achieved coefficients ranging from 0.90 to 0.99 for the regions measured.
Autism Diagnostic Interviews
The Autism Diagnostic Interview—Revised was administered by telephone during separate interviews for each twin by a staff member trained in the reliable administration of this instrument. The Autism Diagnostic Observation Schedule—Generic was administered by the same staff member at our center. Scores were used to diagnose and determine the severity of autism in each subject.
Demographic Variables
In order to further characterize our subjects, the Stanford-Binet Intelligence Scale—Fourth Edition
(46) was administered to the autism and co-twin groups, and the WISC-III
(47) was administered to the comparison group. The comparison group was administered a different IQ test because they were acquired simultaneously for a separate study that necessitated the use of the WISC-III. Socioeconomic status was measured using the Two-Factor Index of Social Standing
(48) .
Statistical Analyses
Analyses of variance (ANOVAs) assessed the effect of study group on volumes of total frontal lobe. Analyses of covariance (ANCOVAs) assessed the effect of study group on volumes of the amygdala and hippocampus, with whole brain volume entered as a covariate. Multiple analyses of covariance (MANCOVAs) assessed the effect of study group on 1) total dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, orbitolateral prefrontal cortex, and orbitomedial prefrontal cortex volumes; 2) the five subregions of the corpus callosum; and 3) the three subregions of the cerebellar vermis. Whole brain volume was entered as a covariate in these analyses. Effect size (ε 2 ) is provided for all analyses of main effects. Intraclass correlation coefficients were used to assess concordance in volumes of regions of interest between twin pairs. Hierarchical regression analyses were conducted in order to determine associations between brain volumes and total scores on both autism diagnostic scales. Since the twin pairs differed significantly in cognitive levels, full-scale IQ was entered into the model initially, followed by volumes of brain regions of interest, all of which were entered as a block.
Results
Sample Characteristics
There were no significant differences between our autism subjects, their discordant co-twins, and the unrelated matched comparison subjects in terms of age or socioeconomic status. All groups differed significantly in IQ scores, as shown in
Table 1 . In terms of categorical levels of intellectual functioning, 62% of participants with autism had IQ scores in the range of mental retardation, 15% in the borderline range, and 23% in the average range. In contrast, 23% of IQ scores among co-twins fell in the range of mental retardation, 23% in the borderline range, and 54% in the average range. Between-twin comparisons of standard subscale scores on the Stanford-Binet Intelligence Scale (
Table 1 ) indicated that twin pairs differed significantly in verbal reasoning (p=0.04), abstract/visual reasoning (p=0.02), and quantitative reasoning (p=0.04) but not short-term memory (p=0.12).
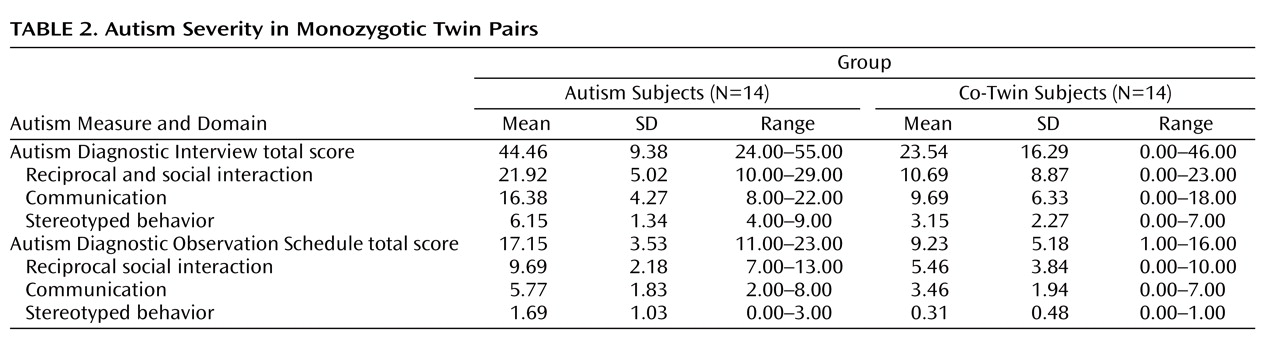
Autism and co-twin groups differed significantly in total scores on both the Autism Diagnostic Interview (F=16.11, df=1, 25, p=0.001) and the Autism Diagnostic Observation Schedule (F=20.75, df=1, 25, p<0.001) (
Table 2 ). Thirty-six percent of the co-twins were diagnosed with autism and therefore were clinically concordant with their twin for autism. Twenty-eight percent of co-twins were diagnosed with pervasive developmental disorder, and 36% had no diagnosis.
Volumetric Comparisons
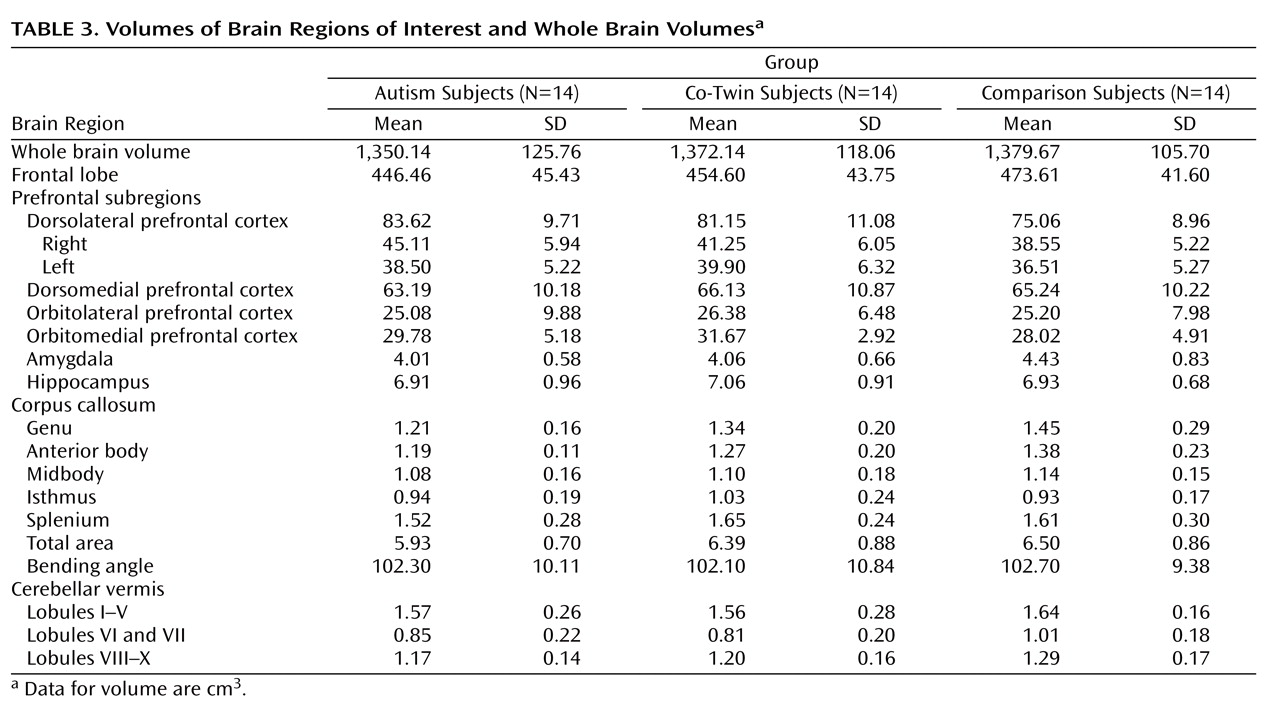
The volumetric means of all regions of interest are shown in
Table 3 . ANOVAs indicated that although children with autism and their co-twins displayed smaller absolute frontal lobe volumes relative to comparison subjects the differences were not significant (F=1.43, df=2, 39, p=0.25), even after covarying for whole brain volume (p=0.28).
A MANCOVA assessing the effects of study group on all prefrontal subregions yielded a Wilks’ lambda distribution of 0.55 (p=0.009). Only dorsolateral prefrontal cortex volumes differed between study groups (F=4.52, df=2, 36, p=0.02; ε 2 =0.20). Whole brain volume was also a significant covariate (p=0.045). Follow-up ANOVAs indicated that the right (but not left) dorsolateral prefrontal cortical volumes for children with autism were significantly larger (F=4.61, df=2, 39, p=0.02; ε 2 =0.191) than those of comparison subjects (p=0.01) but not co-twins (p=0.25).
Corpus callosum areas differed significantly between study groups, yielding a Wilks’ lambda distribution of 0.58 (p=0.047). Only the anterior body and the genu accounted significantly for these differences. Whole brain volume was also a significant covariate (p=0.005). Univariate ANOVAs indicated that genu (F=3.19, df=2, 36, p=0.05; ε 2 =0.15) and anterior body (F=4.18, df=2, 36, p=0.02; ε 2 =0.19) areas were smaller for children with autism relative to comparison subjects but not to co-twins.
The omnibus model for cerebellar vermis volumes was not significant (Wilks’ lambda distribution=0.77, p=0.17). However, univariate analyses indicated that lobules VI and VII differed between study groups (F=3.54, df=2, 38, p=0.04; ε 2 =0.17). Whole brain volume was also a significant covariate. Planned comparisons indicated that relative to comparison subjects lobules VI and VII were significantly smaller in co-twins (p=0.02) and tended to be smaller in children with autism (p=0.06). Amygdala and hippocampus volumes did not differ significantly between study groups.
Neuroanatomic Concordance Between Twin Pairs
Neuroanatomic concordance between twin pairs was assessed with the intraclass correlation coefficient and yielded the following results: whole brain volume, 0.93; frontal lobe, 0.93; dorsolateral prefrontal cortex, 0.65; corpus callosum, 0.78; amygdala, 0.66; hippocampus, 0.91; and cerebellar vermis, 0.82.
Association Between Neuroanatomic Volumes and Severity of Autism
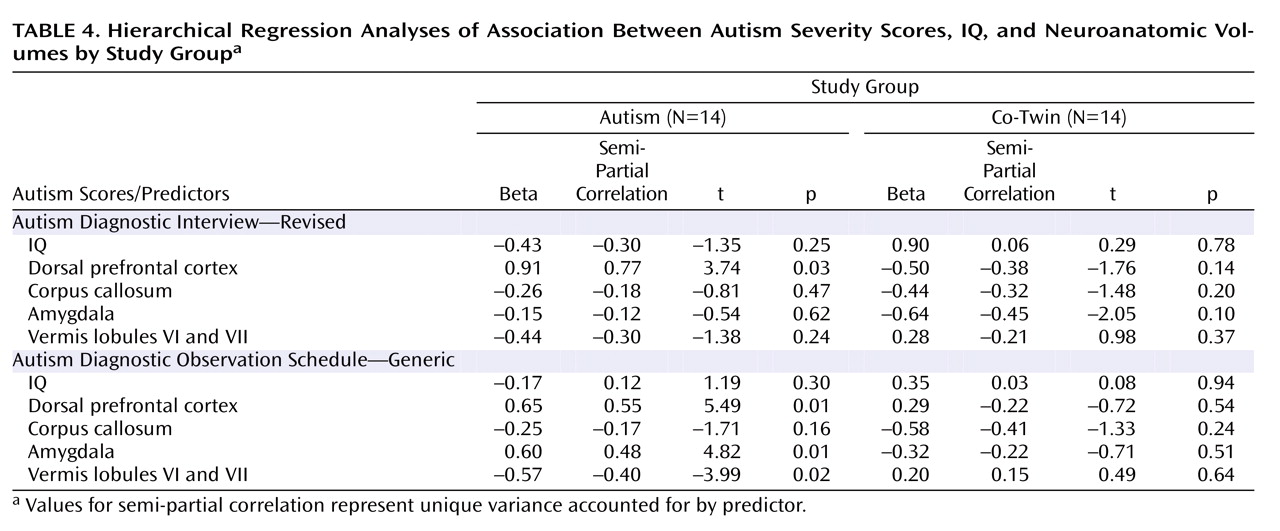
Neuroanatomic regions that significantly differentiated study groups were included in hierarchical regression analyses, including the right dorsolateral prefrontal volumes, anterior body area of the corpus callosum, and vermis lobules VI and VII volumes. Amygdala volumes were also included, since previous studies have reported an association with social and communication deficits in autism
(1,
49) .
Table 4 summarizes these results.
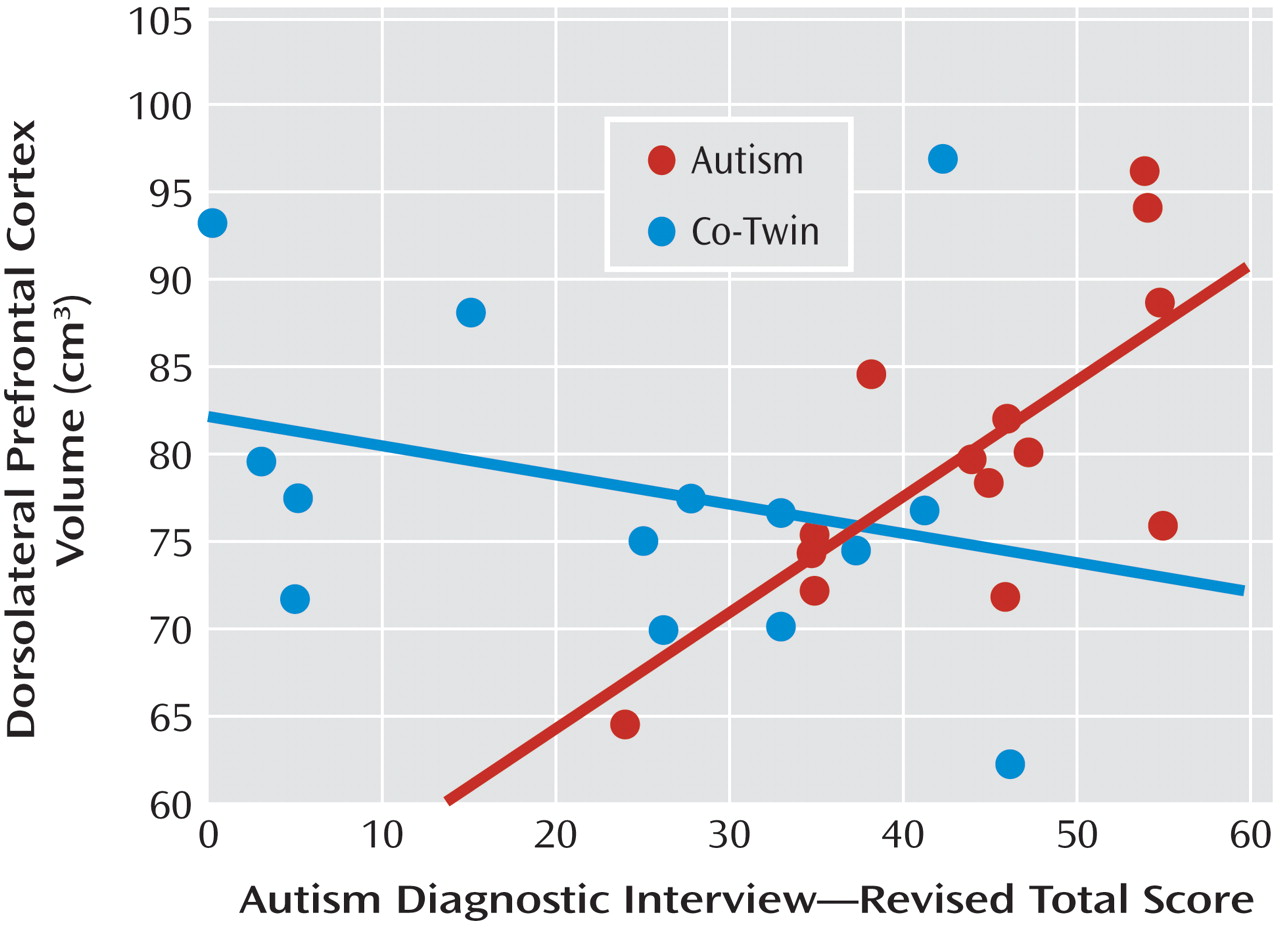
When measured with the Autism Diagnostic Interview, autism severity was not significantly associated with either IQ scores or the block of neuroanatomic volumes for twins with autism or their co-twins. IQ accounted for nearly twice the variance in severity scores in co-twins than it did for twins with autism (28% for co-twins; 15.6% for twins with autism). For both groups, the block of brain volumes accounted for a large proportion of unique variance in severity scores (65% for twins with autism; 48% for co-twins), although only dorsolateral prefrontal volumes in autistic twins were significantly associated with severity scores (
Figure 1 ).
When measured with the Autism Diagnostic Observation Schedule, autism severity was significantly associated with neuroanatomic volumes, but not IQ scores, for twins with autism. Although IQ accounted for only 3% of the variance in autism severity (p=0.66), the combination of brain volumes accounted for 93% of the variance (p=0.005). Larger dorsal prefrontal and amygdala volumes as well as smaller vermis volumes were significant predictors (
Table 4 ). In contrast, this measure of autism severity was not significantly associated with either IQ scores (p=0.47, accounting for 38% of variance) or neuroanatomic volumes (p=0.26, accounting for 14% of variance) for the co-twins, although, again, IQ accounted for a much larger portion of variance in severity for co-twins than for autistic twins.
Discussion
Our study is the first investigation, to our knowledge, of volumetric differences of the prefrontal cortex, corpus callosum, cerebellar vermis, and mesial temporal lobe between monozygotic twins with varying levels of discordance for autism. Relative to comparison subjects, we found larger volumes of the total and right dorsolateral prefrontal cortex and smaller areas of the anterior aspect of the corpus callosum in twins with autism. Neuroanatomic structures of less affected co-twins tended to be midrange between twins with autism and typically developing comparison subjects, with the exception of vermal lobules VI and VII, which were significantly smaller in co-twins than in comparison subjects. Interestingly, the degree of neuroanatomic concordance between twin pairs varied by brain region. In addition, volumes of the dorsal prefrontal cortex, amygdala, and vermal lobules VI and VII accounted for a very large portion of the variance in scores reflecting autism severity for subjects with narrowly defined autism, whereas the same neuroanatomic structures accounted for less variance in autism severity in the co-twins.
Volumetric Differences Among Children With Autism, Co-Twins, and Comparison Subjects
Our findings of increased dorsolateral prefrontal cortical volumes in children with autism are consistent with previous observations of region-specific (as opposed to global) volumetric enlargement of the frontal lobe in young children with autism
(28) . Increases in dorsolateral prefrontal tissue in children with autism may be a result of anomalies in the development of both white matter and synaptic densities
(28), which occur concurrently and in a region-specific manner
(50) . In typically developing children, synapse elimination is most likely complete by age 12 in the auditory cortex, whereas synapse elimination appears to continue until mid-adolescence in the middle frontal gyrus
(50) . Considering the age range of our subjects, our finding of enlarged dorsolateral prefrontal cortical volumes in the absence of hyperplasia of the total frontal lobe may be a result of the likelihood that this region is still developing and has not reached the point of substantial synapse elimination that decreases synaptic density to approximately 60% of maximum
(50) .
Decreases in the genu and anterior body of the corpus callosum are consistent with previous studies that have observed reduced area and fractional anisotropy in the anterior aspects of this structure
(30,
31) . Moreover, the fibers of the anterior portion of the corpus callosum project to the prefrontal cortex
(51), suggesting that the anomalies that we observed in the dorsal prefrontal cortex may be associated with disruptions in connectivity via the corpus callosum. Diffusion tensor imaging studies are needed to specifically identify interprefrontal connectivity of the corpus callosum and the extent to which disruption in those fibers affect dorsal prefrontal morphology
(52) .
Despite evidence for the amygdala’s role in social cognition and “theory of mind”
(1,
53) and studies indicating that the volume of the amygdala is larger in school-age children with autism relative to typically developing children
(7,
54), we did not observe volumetric differences in this structure. Moreover, we did not observe an association between age and volumetric change in the amygdala, which has been reported previously. Our findings may be a result of differences in the age distribution between previous and current research samples or potentially of the effect of twinning on differences in the trajectory of amygdala development.
Lobules VI and VII of the cerebellar vermis have been the subject of numerous morphometric studies in autism since anomalies were initially observed in 1988
(12) . A recent meta-analysis
(55) suggests that heterogeneity of previous findings is related to sample differences in age and IQ. Since the co-twins in our sample exhibited reductions of lobules VI and VII to a greater extent than twins with autism, who had lower IQ scores, our findings do not support the notion
(55) that group differences in cerebellar vermis area decrease as age and IQ increase. Nonetheless, the moderate vermis reductions that we detected in co-twins and their more severely affected twins suggest that disruptions to the vermis may be associated with both the narrow and the broader phenotype for autism, whereas disruptions in cerebral structures may be associated with the narrow phenotype only.
Neuroanatomic Concordance Within Twin Pairs
Recent studies
(36,
56) of typically developing and adult monozygotic twins have observed a high degree of within-pair neuroanatomic concordance, with correlations of at least 0.80. In contrast, the intraclass correlation coefficients reported in the present study range from 0.65 to 0.93. The variability that we observed in the degree of neuroanatomic concordance may be a result of differences in maturational trajectories in the regions of the brain that we measured. The dorsal prefrontal cortex, amygdala, and corpus callosum mature relatively late in typically developing individuals
(57) . Moreover, age-related variation in the amygdala
(54) and the dorsal prefrontal cortex
(2) has been noted in autism. In addition, variability in concordance may be a function of differences in plasticity of specific brain regions. For example, evidence of both experience-dependent cortical plasticity
(58,
59) and of protracted development of the prefrontal cortex
(50) would suggest that in early childhood the less-affected co-twins may have had the capacity and resources to interact with environmental stimuli in a manner that supported more typical neural development of the prefrontal cortex. Future longitudinal studies of discordant monozygotic twin pairs that investigate neuroanatomic variations over time are needed to further delineate neuroanatomic growth patterns and behavioral trajectories in children with autism and their co-twins.
Association Between Volumes and Behavioral Phenotypes
Our findings are consistent with previous studies reporting associations between the prefrontal cortex, amygdala, and cerebellar vermis volumes and the core features of autism. One caveat in studies of associations between prefrontal volumes and autism features is that they vary in the specific substructures of the prefrontal cortex that are associated with social and communication deficits in children with autism
(60) . In addition, both positive and negative
(61) associations between social interaction/communication and the prefrontal cortex have been observed, although this difference may be a result of between-study differences in the age range of participants.
Both positive and negative associations have also been observed between amygdala volumes and comorbid/behavioral features of autism, including a positive association between amygdala volumes and the degree of anxiety
(49) and social and communication deficits
(1) and a negative association between amygdala volumes and social reciprocity
(10) . Again, differences in the age and degree of impairment between samples may account for these inconsistencies. Taken together, however, these findings support the role that the amygdala plays in the behavioral phenotype of individuals with autism.
Associations between decreases in lobules VI and VII of the cerebellar vermis and stereotyped and restrictive behaviors have been reported in children with autism
(62) and in children with fragile X syndrome, a disorder that can include core features of autism
(63) . Through its connections to the brainstem and cortical areas involved in auditory processing and attention
(64 –
66), one function of the vermis is to mediate sensory stimuli
(64,
67) . Accordingly, disruptions in the connectivity of these circuits may underlie the association between decreases in this structure and severity of autistic features.
Notably, full-scale intelligence did not account for a significant portion of the variance in either of the autism severity scores for the twins with autism or their co-twins. Nonetheless, in co-twins, IQ scores accounted for a much greater proportion of variance in autism severity than in the twins with autism. These differences could be a result of the small sample size and restricted ranges of either IQ scores in the twins with autism or neuroanatomic volumes in the co-twins, all affecting power to detect associations. However, this finding may also support the notion of what Rutter and colleagues
(68) referred to as a “second hit,” which leads to the development of a severe autism disorder above and beyond the risk for autism that may be conferred by either genetic vulnerability or the presence of intellectual disability
(67) . Potentially, a “second hit” could consist of epigenetic and/or nonshared environmental events that moderate genetic contributions to the behavioral phenotypes of our monozygotic twins. Potentially, the monozygotic twins in our study have different DNA methylation patterns or other epigenetic differences, leading to varying environmental stimuli susceptibilities
(67) and dissimilar regulation of gene expression and, ultimately, discordant behavioral phenotypes.
Limitations and Conclusions
It is important to note that the twinning process may have affected our results. To the extent that premature births or lower birth weights characterize twins to a greater extent than singletons, the neuroanatomic abnormalities observed in our autism twins may be different from those that would occur in singleton subjects with autism disorder. Accordingly, our study would be strengthened by the inclusion of monozygotic twin pairs as comparison subjects. Moreover, in order to disentangle the genetic versus shared and nonshared environmental influences that contribute to neuroanatomic abnormalities in children with autism, future studies should include both typically developing monozygotic twins as well as dizygotic twins in which at least one twin is affected with autism. The present study is further limited by the small size and large age range of the sample (which reduced power to detect other neuroanatomic differences), the utilization of different IQ measures for the twin pairs and comparison subjects, and the lack of social functioning scores for all subjects, preventing us from investigating the association between brain regions of interest and social and communication deficits across all subjects. Finally, to the extent that alterations in the frontal lobe occur during the most critical time of language and social development, the incorporation of a longitudinal design in future studies is also essential. Despite these limitations, our study replicates previous findings of an enlarged dorsolateral prefrontal cortex, reduced areas of the corpus callosum, and decreased posterior cerebellar vermis in autism and demonstrates that neuroanatomic alterations are closely associated with phenotypic features of autism in children who are severely affected.