Recurrent mood episodes are a hallmark of bipolar disorder, with as many as 50% of patients experiencing recurrence within 1 year after resolution of an acute episode
(1 –
4) . Multiple pharmacotherapies, including lithium and lamotrigine as well as some antipsychotic medications, have demonstrated efficacy in delaying recurrence of mood episodes in bipolar disorder
(5 –
9) . However, the molecular mechanisms by which these drugs exert their therapeutic effects have not been established, despite intensive study implicating multiple pathways
(10), particularly for lithium.
Some evidence suggests that response to lithium treatment is familial
(11) and associated with a family history of bipolar disorder
(12) . However, initial candidate gene investigations of lithium response have not yet yielded replicated findings
(13 –
15) . Limited understanding of the mechanism of action of bipolar pharmacotherapies impedes further candidate gene association studies and argues for a more unbiased genomewide approach. Indeed, recent transcriptional profiling studies
(16,
17) have identified changes following lithium treatment in genes not previously connected with lithium response.
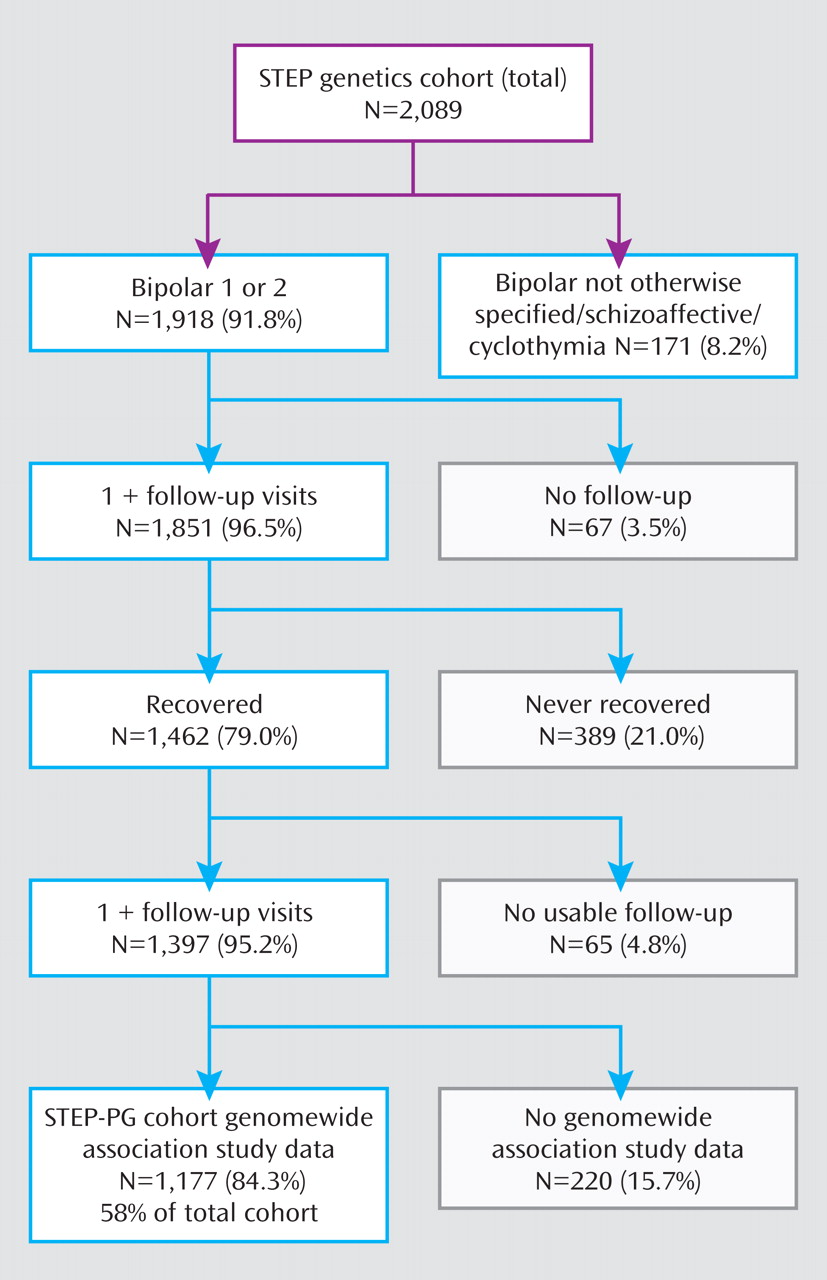
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study
(3,
18) was an effectiveness study that examined real-world outcomes in a large cohort of patients receiving guideline-based, nonrandomized treatment. Results from a genomewide association study of the STEP-BD cohort have recently been reported
(19,
20) . We utilized these genomewide data to examine association with risk for recurrence among patients treated with lithium. We then examined the regions that showed the greatest evidence of association in a second cohort of bipolar I and II disorder patients who were drawn from clinical populations at University College London.
Discussion
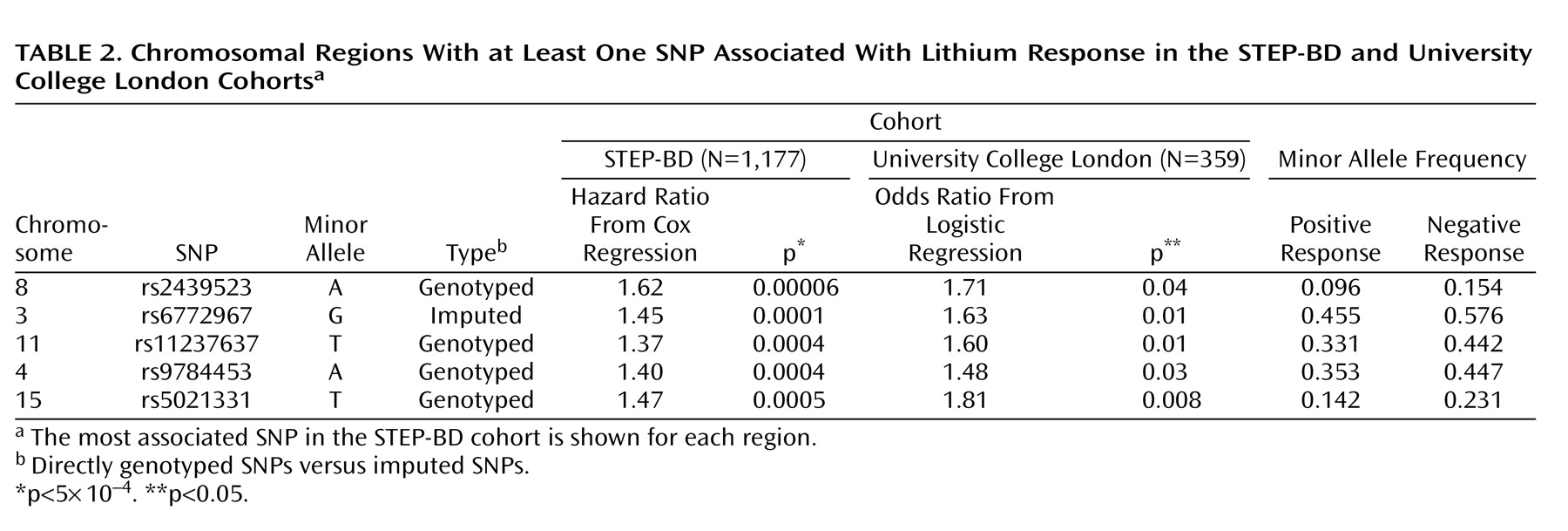
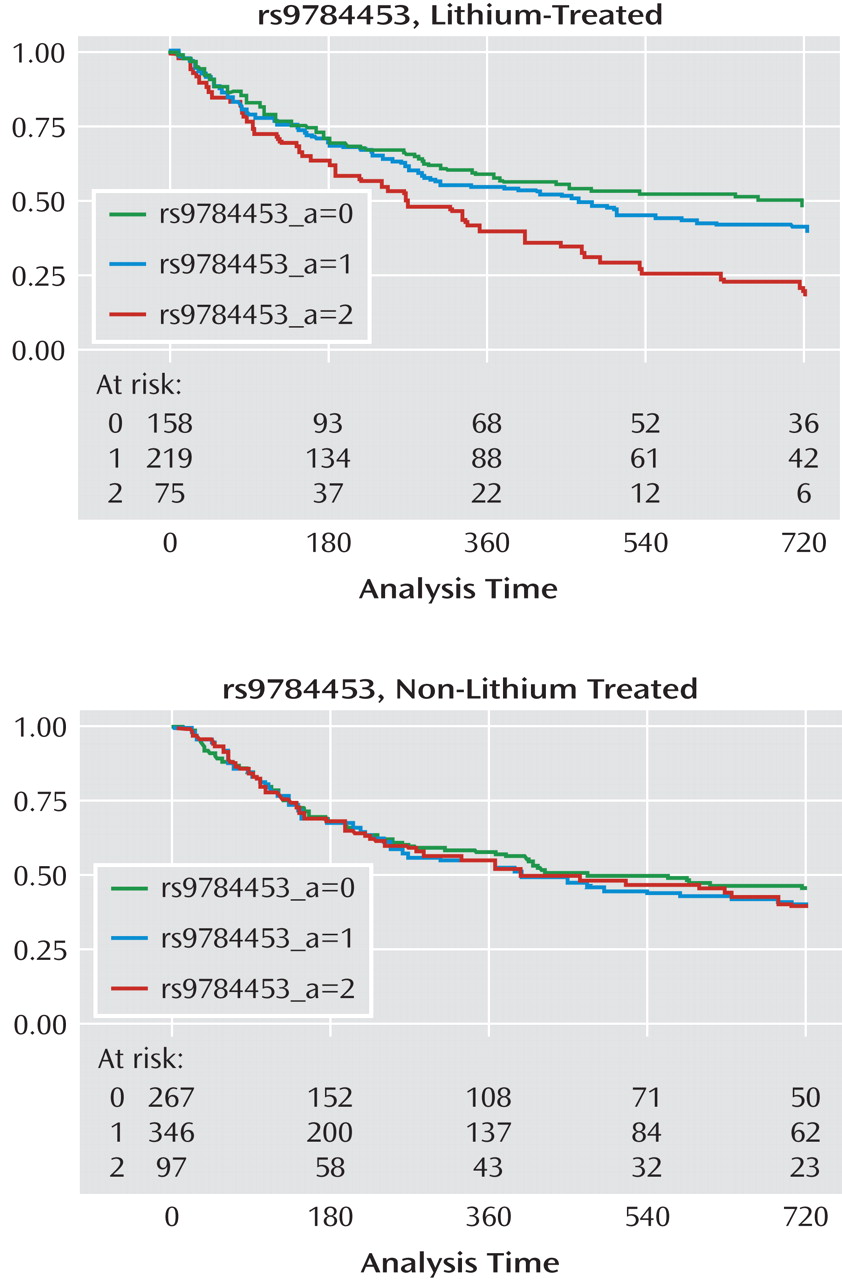
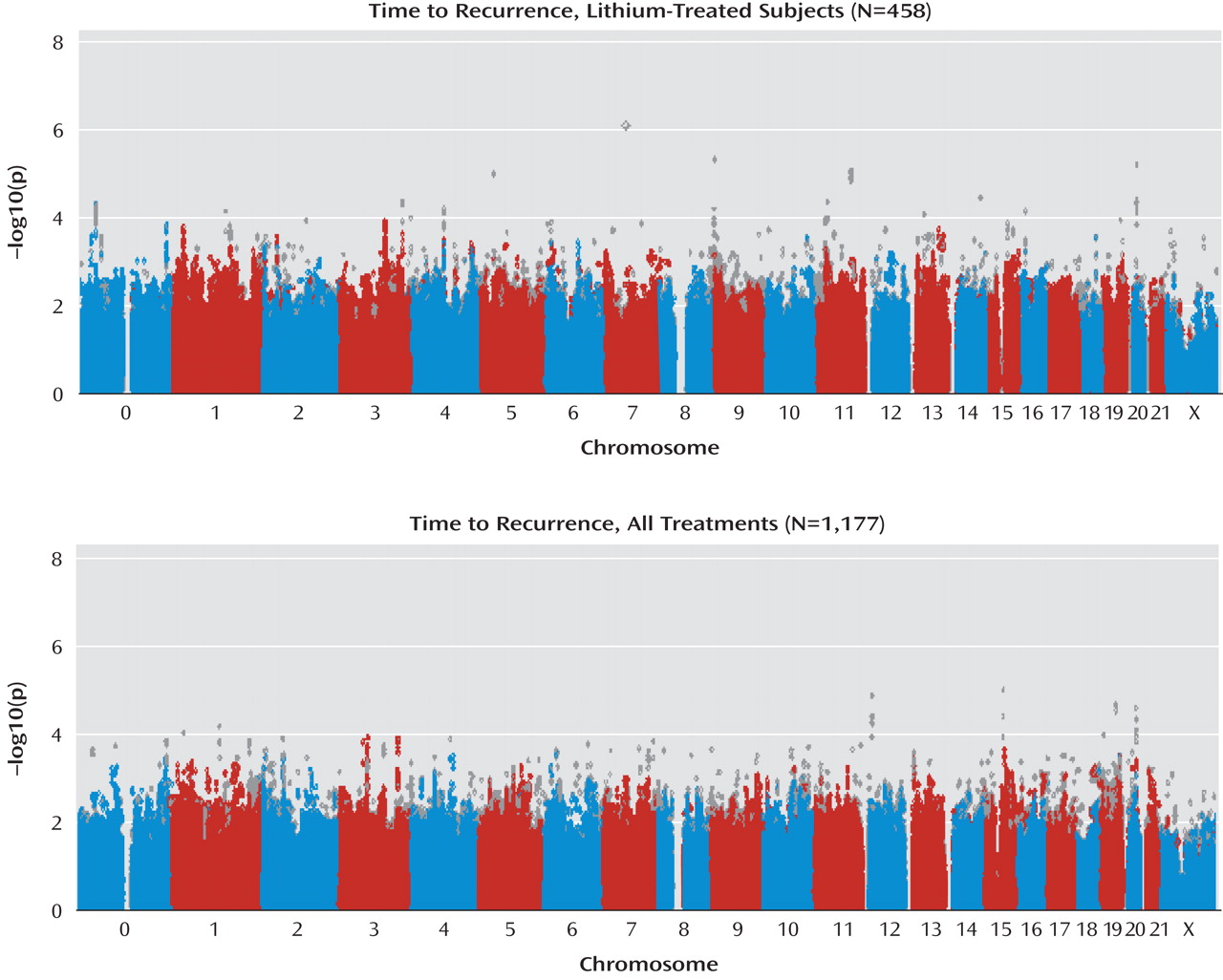
In the present study, which to our knowledge is the first genomewide association study of lithium response in bipolar disorder, no SNP met the threshold for genomewide association. However, these results may still be useful in laying the groundwork for subsequent genetic and proteomic investigations by helping to prioritize association results. Of the regions with a p value of <5×10
–4 in the STEP-BD cohort, five showed consistent evidence of association in a second cohort of lithium-treated patients (University College London cohort). Among these five regions is the gene coding for a subunit of the ligand-gated ionotropic glutamate receptor, GluR2/GLURB, which binds to alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate (AMPA). Notably, a recent report suggested that
GRIA2 is one of the genes downregulated by chronic lithium treatment in a human neuronal cell line
(17), and chronic lithium or valproate treatment causes decreased synaptic expression of GluR2 in hippocampal neurons
(29) . In a magnetic resonance spectroscopy study, hippocampal glutamate concentrations were significantly increased in euthymic chronically lithium-treated patients relative to healthy comparison subjects
(30) . Thus, our results add to a convergent body of evidence suggesting the importance of glutamate in bipolar disorder and the mechanism by which lithium may affect glutamatergic neurotransmission.
Among the other regions with consistent association in the STEP-BD and University College London cohorts were those containing 1) Syndecan-2 (
SDC2 ), which codes for a cell-surface proteoglycan that has been shown to play a central role in the formation of dendritic spines in the hippocampus
(31) ; 2) synaptic vesicle glycoprotein-2B (
SV2B ), a protein of unknown function expressed primarily in the hippocampus
(32) ; and 3) the human homologue of Drosophila odd Oz (odz)-4 (
ODZ4 ), implicated in brain patterning
(33) . Taken together our results also suggest that response to lithium is likely influenced by multiple genes of modest effect, similar to complex genetic diseases themselves.
We did not find significant evidence of association with treatment response for variation in regions previously implicated in a meta-analysis of genomewide association studies of bipolar disorder
(20) . This is perhaps not surprising given that the genes in these regions account for a very small proportion of attributable risks of bipolar disorder. However, we cannot exclude the possibility that lithium acts up- or downstream of these genes.
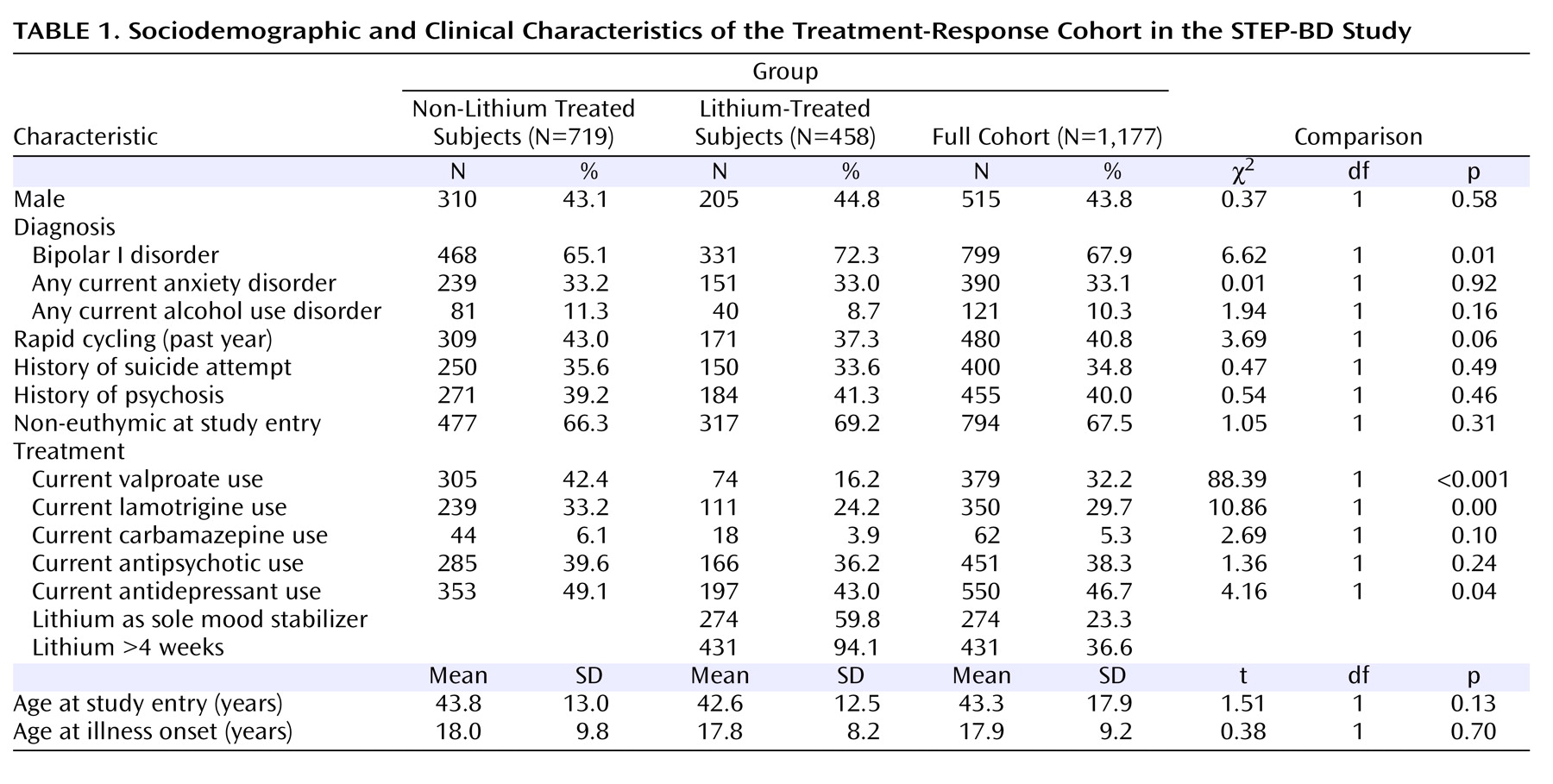
The STEP-BD cohort presents both limitations and strengths for pharmacogenetic study. A primary limitation is the absence of detailed retrospective data to allow contrast between pre- and posttreatment course. As a result, we were unable to utilize the criteria for good versus poor lithium response, as developed by Grof and colleagues
(11), which relies on knowledge of prelithium course. Further limitations of the STEP-BD cohort are that treatments were not always initiated at the onset of a mood episode, treatment assignment was not randomized, and medications were often administered in combination. These features make assignment of longer-term outcomes to a given medication complex. Of note, this is a problem in bipolar disorder clinical practice as well, in which it can be difficult for the clinician and patient to “assign” benefit to a given pharmacotherapy, particularly if it is part of a combination regimen. Therefore, we made simplifying assumptions based on when clinicians typically initiate and discontinue new treatments
(5) . Specifically, for time-to-recurrence, we assumed that if a patient was receiving a treatment at baseline, an outcome could be assigned to that medication. This approach corresponds to intent-to-treat in clinical trials. We did not require a specific threshold lithium level, since trough levels were not available for the majority of subjects, although STEP-BD clinician education in evidence-based practice included the importance of adequate lithium dosing.
On the other hand, the STEP-BD study has advantages for pharmacogenetic analysis. First, because of the availability of detailed prospective assessments, our confidence in the reliability of outcomes is higher than that available from typical retrospective assessment. Second, the STEP-BD study’s effectiveness design should yield greater generalizability and ecological validity than, for example, randomized controlled trials. Such trials typically exclude individuals with anxiety or substance use comorbidity, despite the prevalence of these comorbidities in clinical practice
(34) . Finally, the total number of patients with time to recurrence available far exceeds that of other reported cohorts to date, and the availability of multiple treatment groups allows for examination of overlap in association.
In addition, little is known about the homogeneity of response between bipolar I disorder and bipolar II disorder
(35), but modern studies suggest similar efficacy for lithium in both subgroups
(3,
5,
35) . We therefore elected to pool these two groups but adjust our associations by bipolar subtype. Among the SNPs showing the greatest evidence of association with lithium response, we did not detect evidence of gene-by-bipolar subtype interactions.
A notable feature of the present analysis is the availability of a replication cohort. The optimal replication cohort would have allowed the examination of an identical phenotype (i.e., time to recurrence). A quantitative survival phenotype as such should yield greater statistical power than deriving a categorical outcome. However, to our knowledge, no such cohort is extant. Alternatively, we could have derived a “positive response” phenotype more similar to that of the University College London cohort or other lithium-response cohorts. We elected not to categorize time to recurrence outcomes for the STEP-BD cohort in terms of “good” or “poor” response because to do so would have entailed an arbitrary distinction. Still, the lack of correspondence between the responsiveness phenotype in the two cohorts suggests that failure to “replicate” should be interpreted cautiously.
Taken together, our results do suggest a number of regions meriting further investigation. They further highlight the importance of collecting adequate replication cohorts with detailed longitudinal outcomes if the effect of genetic variation on lithium response is to be understood. An international consortium for the study of lithium genetics will be useful in this regard (http://www.conligen.org/). Finally, our results suggest the potential utility of pharmacogenetic investigation in bipolar disorder for elucidating mechanisms of action of established mood stabilizing medications.